Ritalin
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Ritalin, Concerta, Aptensio, Biphentin, Daytrana, Equasym, Medikinet, Metadate, Methylin, QuilliChew, Quillivant |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682188 |
| License data |
|
| Pregnancy category |
|
| Dependence liability |
Physical: None Psychological: Moderate |
| Addiction liability |
Moderate |
| Routes of administration |
Oral, insufflation, intravenous, transdermal |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ~30% (range: 11–52%) |
| Protein binding | 10–33% |
| Metabolism | Liver (80%) mostly CES1A1-mediated |
| Biological half-life | 2–3 hours |
| Excretion | Urine (90%) |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.003.662 |
| Chemical and physical data | |
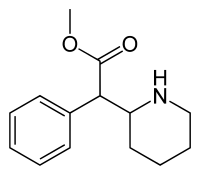
| Formula | C14H19NO2 |
| Molar mass | 233.31 g/mol |
| 3D model (Jmol) | |
| Melting point | 74 °C (165 °F) |
| Boiling point | 136 °C (277 °F) |
|
|
|
|
Methylphenidate, sold under various trade names, Ritalin being one of the most commonly known, is a central nervous system (CNS) stimulant of the phenethylamine and piperidine classes that is used in the treatment of attention deficit hyperactivity disorder (ADHD) and narcolepsy. The original patent was owned by CIBA, now Novartis Corporation. It was first licensed by the US Food and Drug Administration (FDA) in 1955 for treating what was then known as hyperactivity.
Medical use began in 1960; the drug has become increasingly prescribed since the 1990s, when the diagnosis of ADHD became more widely accepted. Between 2007 and 2012 methylphenidate prescriptions increased by 50% in the United Kingdom and in 2013 global methylphenidate consumption increased to 2.4 billion doses, a 66% increase from the year before. The United States continues to account for more than 80% of global consumption.
ADHD and other similar conditions are believed to be linked to sub-performance of the dopamine and norepinephrine functions in the brain, primarily in the prefrontal cortex, responsible for executive function (e.g., reasoning, inhibiting behaviors, organizing, problem solving, planning, etc.). Methylphenidate's mechanism of action involves the inhibition of catecholamine reuptake, primarily as a dopamine reuptake inhibitor. Methylphenidate acts by blocking the dopamine transporter and norepinephrine transporter, leading to increased concentrations of dopamine and norepinephrine within the synaptic cleft. This effect in turn leads to increased neurotransmission of dopamine and norepinephrine. Methylphenidate is also a weak 5HT1A receptor agonist.
...
Wikipedia
