Retroviral ribonuclease H
| Retroviral ribonuclease H | |||||||||
|---|---|---|---|---|---|---|---|---|---|
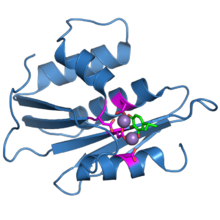
The ribonuclease H domain from the HIV-1 reverse transcriptase protein. The four active-site carboxylate residues are shown in magenta. Two bound manganese ions are shown as purple spheres. A bound inhibitor molecule, beta-thujaplicinol, is shown in green.
|
|||||||||
| Identifiers | |||||||||
| EC number | 3.1.26.13 | ||||||||
| CAS number | 9050-76-4 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
|
|||||||||
| Search | |
|---|---|
| PMC | articles |
| PubMed | articles |
| NCBI | proteins |
The retroviral ribonuclease H (retroviral RNase H) is a catalytic domain of the retroviral reverse transcriptase (RT) enzyme. The RT enzyme is used to generate complementary DNA (cDNA) from the retroviral RNA genome. This process is called reverse transcription. To complete this complex process, the retroviral RT enzymes need to adopt a multifunctional nature. They therefore possess 3 of the following biochemical activities: RNA-dependent DNA polymerase, ribonuclease H, and DNA-dependent DNA polymerase activities ). Like all RNase H enzymes, the retroviral RNase H domain cleaves DNA/RNA duplexes and will not degrade DNA or unhybridized RNA.
Although the RT structures from human, murine and avian retroviruses display different subunits, the relative sizes, orientation and connection of the DNA polymerase and RNase H domains are strikingly similar. The RNase H domain occupies ~25% of the RT protein C-terminal. The DNA polymerase domain occupies ~55% of the RT protein N-terminal. The RNase H domains of MMLV and HIV-1 RT enzymes are structural very similar to the Escherichia coli and Bacillus halodurans RNases H as well as to human RNaseH1. In general, the folded structures of retroviral RNase H domains take the form of 5-stranded mixed beta sheets flanked by four alpha helices in an asymmetric distribution. A notable difference between the various RNase H proteins is the presence or absence of the C-helix (present in E. coli, MLV and human RNases H, absent in HIV-1, B. halodurans and ASLV RNases H), a positively charged alpha helix also referred to as the basic loop or protrusion. It is believed to have a role in substrate binding.
...
Wikipedia
