Rasagiline
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Azilect |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a606017 |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | N04BD02 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 36% |
| Protein binding | 88 – 94% |
| Metabolism | Hepatic (CYP1A2-mediated) |
| Biological half-life | 3 hours |
| Excretion | Renal and fecal |
| Identifiers | |
|
|
| CAS Number |
136236-51-6 |
| PubChem (CID) | 3052776 |
| IUPHAR/BPS | 6641 |
| DrugBank |
DB01367 |
| ChemSpider |
2314553 |
| UNII |
003N66TS6T |
| KEGG |
D02562 |
| ChEMBL |
CHEMBL887 |
| PDB ligand ID | RAS (PDBe, RCSB PDB) |
| Chemical and physical data | |
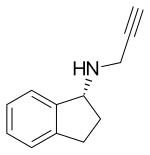
| Formula | C12H13N |
| Molar mass | 171.238 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
|
|
|
Rasagiline (Azilect, TVP-1012, N-propargyl-1(R)-aminoindan) is an irreversible inhibitor of monoamine oxidase-B used as a monotherapy to treat symptoms in early Parkinson's disease or as an adjunct therapy in more advanced cases.
The racemic form of the drug was invented by Aspro Nicholas in the early 1979s. Moussa B.H. Youdim identified it as a potential drug for Parkinson's disease, and working with collaborators at Technion – Israel Institute of Technology in Israel and the drug company, Teva Pharmaceutical, identified the R-isomer as the active form of the drug. Teva brought it to market in partnership with Lundbeck in Europe and Eisai in the US and elsewhere. It was approved in Europe in 2005 and in the US in 2006.
Rasagiline is used to treat symptoms of Parkinson's disease both alone and in combination with other drugs. It has shown efficacy in both early and advanced Parkinsons, and appears to be especially useful in dealing with non-motor symptoms like fatigue.
Rasagiline has not been tested in pregnant women and is Pregnancy Category C in the US.
The FDA label contains warnings that rasagiline may cause severe hypertension or hypotension, may make people sleepy, may make motor control worse in some people, may cause hallucinations and psychotic-like behavior, may cause impulse control disorder, may increase the risk of melanoma, and upon withdrawal may cause high fever or confusion.
Side effects when the drug is taken alone include flu-like symptoms, joint pain, depression, stomach upset, headache, dizziness, and insomnia. When taken with L-DOPA, side effects include increased movement problems, accidental injury, sudden drops in blood pressure, joint pain and swelling, dry mouth, rash, abnormal dreams and digestive problems including vomiting, loss of appetite, weight loss, abdominal pain, nausea, constipation. When taken with Parkinson's drugs other than L-DOPA, side effects include peripheral edema, fall, joint pain, cough, and insomnia.
...
Wikipedia
