Proton sponge
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
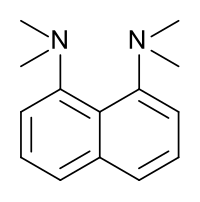
N,N,N',N'-tetramethylnaphthalene-1,8-diamine
|
|
| Other names
Proton Sponge
|
|
| Identifiers | |
|
20734-58-1 |
|
| 3D model (Jmol) |
Interactive image Interactive image |
| ChemSpider |
80012 |
| ECHA InfoCard | 100.039.986 |
| PubChem | 88675 |
|
|
|
|
| Properties | |
| C14H18N2 | |
| Molar mass | 214.31 g·mol−1 |
| Melting point | 47.8 °C (118.0 °F; 320.9 K) |
| Acidity (pKa) | 12.1 (in water) 18.62 (in acetonitrile) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
18.62 (in acetonitrile)
(acidity of the conjugate acid C14H18N2H+)
1,8-Bis(dimethylamino)naphthalene is an organic compound with the formula C10H6(NMe2)2 (Me = methyl). It is classified as a peri-naphthalene, i.e. a 1,8-disubstituted derivative of naphthalene. Owing to its unusual structure, it exhibits exceptional basicity. It is a colorless liquid. It is often referred by the trade name Proton Sponge, a trademark of Sigma-Aldrich.
This compound is a diamine in which the two dimethylamino groups are attached on the same side or peri position of a naphthalene system. Proton-sponge has several very interesting properties; one is its very high basicity; another is its spectroscopic properties.
With a pKa of 12.34 for its conjugate acid in aqueous solution, 1,8-bis(dimethylamino)naphthalene is one of the strongest amine bases. It only absorbs protons slowly—hence the trade name. The high basicity is attributed to the relief of strain upon protonation and/or the strong interaction between the nitrogen lone pairs. It is sterically hindered, making it a weak nucleophile. Because of this combination of properties, it has been used in organic synthesis as a highly selective non-nucleophilic base.
This compound is commercially available. It may be prepared by the methylation of 1,8-diaminonaphthalene with iodomethane or dimethyl sulfate.
...
Wikipedia
