Potassium t-butoxide
|
|
|||
 |
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
potassium 2-methylpropan-2-olate
|
|||
| Identifiers | |||
|
3D model (Jmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.011.583 | ||
|
PubChem CID
|
|||
|
|||
|
|||
| Properties | |||
| C4H9KO | |||
| Molar mass | 112.21 g mol−1 | ||
| Appearance | solid | ||
| Melting point | 256 °C (493 °F; 529 K) | ||
| Solubility in diethyl ether | 4.34 g/100 g (25-26 °C) | ||
| Solubility in Hexane | 0.27 g/100 g (25-26 °C) | ||
| Solubility in Toluene | 2.27 g/100 g (25-26 °C) | ||
| Solubility in THF | 25.00 g/100 g (25-26 °C) | ||
| Hazards | |||
| Safety data sheet | Oxford MSDS | ||
|
EU classification (DSD)
|
Harmful (Xn), Corrosive (C) | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
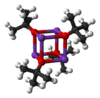
Potassium tert-butoxide is the chemical compound with the formula K+(CH3)3CO−. This colourless solid is a strong base (pKa of conjugate acid around 17) which is useful in organic synthesis. It exists as a tetrameric cubane-type cluster. It is often seen written in chemical literature as potassium t-butoxide.
Potassium t-butoxide is commercially available as a solution and as a solid, but it is often generated in situ for laboratory use because samples are so sensitive and older samples are often of poor quality. It is prepared by the reaction of dry tert-butyl alcohol with potassium metal. The solid is obtained by evaporating these solutions followed by heating the solid. The solid can be purified by sublimation at 220 °C and 1 mmHg.
Potassium tert-butoxide crystallises from THF/pentane at −20 °C as [tBuOK·tBuOH]∞, which consists of infinite one-dimensional chains linked by hydrogen bonding. Sublimation of [tBuOK·tBuOH]∞ affords the tetramer [tBuOK]4, which adopts a cubane-like structure. Mild Lewis basic solvents such as THF and diethyl ether do not break up the tetrameric structure, which persists in the solid, in solution and even in the gas phase.
The tert-butoxide species is itself useful as a strong, non-nucleophilic base in organic chemistry. It is not as strong as amide bases, e.g. lithium diisopropylamide, but stronger than potassium hydroxide. Its steric bulk inhibits the group from participating in nucleophilic addition, such as in a Williamson ether synthesis or an SN2 reaction. Substrates that are deprotonated by potassium t-butoxide include terminal acetylenes and active methylene compounds. It is useful in dehydrohalogenation reactions.
...
Wikipedia