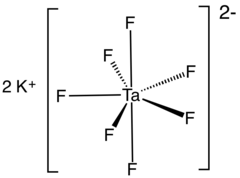
Potassium heptafluorotantalate
 |
|
| Names | |
|---|---|
|
IUPAC name
Dipotassium heptafluorotantalate
|
|
|
Systematic IUPAC name
Dipotassium heptafluorotantalum(2-)
|
|
| Other names
Potassium heptafluorotantalate(V)
Potassium fluorotantalate |
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.037.245 |
| EC Number | 240-986-1 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| K2[TaF7] | |
| Molar mass | 392.13 g/mol |
| Appearance | white solid |
| Density | 4.56 g/mL at 25 °C |
| Melting point | 630 to 820 °C (1,166 to 1,508 °F; 903 to 1,093 K) |
| 0.5 g/100 mL (15 °C) | |
| Hazards | |
| GHS pictograms |
 
|
| GHS signal word | Danger |
| H301, H315, H319, H331, H335 | |
| P261, P264, P270, P271, P280, P301+310, P302+352, P304+340, P305+351+338, P311, P312, P321, P330, P332+313, P337+313, P362, P403+233, P405, P501 | |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose)
|
110 mg/kg (Oral: rat) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Potassium heptafluorotantalate is an inorganic compound with the formula K2[TaF7]. It is the potassium salt of the heptafluorotantalate anion [TaF7]2−. This white, water-soluble solid is an intermediate in the purification of tantalum from its ores and is the precursor to the metal.
Potassium heptafluorotantalate is an intermediate in the industrial production of metallic tantalum. Its production involves leaching tantalum ores, such as columbite and tantalite, with hydrofluoric acid and sulfuric acid to produce the water-soluble hydrogen pentafluorotantalate.
This solution is subjected to a number of liquid-liquid extraction steps to remove metallic impurities (most importantly niobium) before being treated with potassium fluoride to produce K2[TaF7]
Hydrofluoric acid is both corrosive and toxic, making it unappealing to work with; as such a number of alternative processes have been developed for small-scale syntheses. Potassium heptafluorotantalate can be produced by both anhydrous and wet methods. The anhydrous method involves the reaction of tantalum oxide with potassium bifluoride or ammonium bifluoride according to the following equation:
The method was originally reported by Berzelius.
K2[TaF7] can also be precipitated from solutions in hydrofluoric acid provided that the concentration of HF is below about 42%. Solutions having higher concentrations of HF yield potassium hexafluorotantalate [KTaF6]. The K-salt can be also precipitated from a solution in hydrofluoric acid of tantalum pentachloride:
...
Wikipedia
