Tantalum pentachloride
 |
|
| Names | |
|---|---|
|
IUPAC names
Tantalum(V) chloride
Tantalum pentachloride |
|
| Identifiers | |
|
|
|
3D model (Jmol)
|
|
| ECHA InfoCard | 100.028.869 |
| EC Number | 231-755-6 |
|
PubChem CID
|
|
| UNII | |
|
|
|
|
| Properties | |
| TaCl5 | |
| Molar mass | 358.213 g/mol |
| Appearance | white monoclinic crystals |
| Density | 3.68 g/cm3 |
| Melting point | 216 °C (421 °F; 489 K) |
| Boiling point | 239.4 °C (462.9 °F; 512.5 K) (decomposes) |
| reacts | |
| Solubility | soluble in ethanol, ether, CCl4 |
| +140.0·10−6 cm3/mol | |
| Structure | |
| Monoclinic, mS72 | |
| C2/m, No. 12 | |
| Thermochemistry | |
|
Std molar
entropy (S |
221.75 J K−1 mol−1 |
|
Std enthalpy of
formation (ΔfH |
-858.98 kJ/mol |
| Hazards | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose)
|
1900 mg/kg (oral, rat) |
| Related compounds | |
|
Other anions
|
Tantalum(V) fluoride Tantalum(V) bromide Tantalum(V) iodide |
|
Other cations
|
Vanadium(IV) chloride Niobium(V) chloride |
|
Related compounds
|
Tantalum(III) chloride, Tantalum(IV) chloride |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Tantalum(V) chloride, also known as tantalum pentachloride, is an inorganic compound with the formula TaCl5. It takes the form of a white powder and is commonly used as a starting material in tantalum chemistry. It readily hydrolyzes to form tantalum(V) oxychloride (TaOCl3) and eventually tantalum pentoxide (Ta2O5); this requires that it be synthesised and manipulated under anhydrous conditions, using air-free techniques.
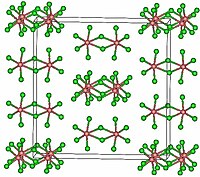
TaCl5 crystallizes in the monoclinic space group C2/m. The ten chlorine atoms define a pair of octahedra that share a common edge. The tantalum atoms occupy the centres of the octahedra and are joined by two chlorine-bridging ligands. The dimeric structure is retained in non-complexing solvents and to a large extent in the molten state. In the vapour state, however, TaCl5 is monomeric. This monomer adopts trigonal bipyramidal structure, like that of PCl5.
The solubility of tantalum pentachloride increases to a slightly for the following series of aromatic hydrocarbons: benzene< toluene< m-xylene< mesitylene, as reflected in the deepening of colour of the solutions from pale yellow to orange. Tantalum pentachloride is less soluble in cyclohexane and carbon tetrachloride than in the aromatic hydrocarbons. Such solutions of tantalum pentachloride is also known to be a poor conductor of electricity, indicating little ionization. TaCl5 is purified by sublimation to give white needles.
Tantalum pentachloride can be prepared by reacting powdered, metallic tantalum with chlorine gas at between 170 - 250 °C. This reaction can also be performed using HCl at 400 °C.
It can also be prepared by a reaction between tantalum pentoxide and thionyl chloride at 240 °C
...
Wikipedia
