Potassium ferricyanide
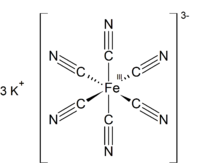 |
|
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Potassium hexacyanoferrate(III)
|
|
| Other names
Red prussiate of Potash,
Prussian red, Potassium ferricyanide |
|
| Identifiers | |
|
13746-66-2 |
|
| 3D model (Jmol) | Interactive image |
| ChemSpider |
24458 |
| ECHA InfoCard | 100.033.916 |
| PubChem | 26250 |
| RTECS number | LJ8225000 |
|
|
|
|
| Properties | |
| K3[Fe(CN)6] | |
| Molar mass | 329.24 g/mol |
| Appearance | deep red crystals, sometimes small pellets, orange to dark red powder |
| Density | 1.89 g/cm3, solid |
| Melting point | 300 °C (572 °F; 573 K) |
| Boiling point | decomposes |
| 330 g/L ("cold water") 464 g/L (20 °C) 775 g/L ("hot water") |
|
| Solubility | slightly soluble in alcohol soluble in acid soluble in water |
| +2290.0·10−6 cm3/mol | |
| Structure | |
| monoclinic | |
| octahedral at Fe | |
| Hazards | |
| Safety data sheet | MSDS |
| R-phrases | R20, R21, R22, R32 |
| S-phrases | S26, S36 |
| Flash point | Non-flammable |
| Related compounds | |
|
Other anions
|
Potassium ferrocyanide |
|
Other cations
|
Prussian blue |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Potassium ferricyanide is the chemical compound with the formula K3[Fe(CN)6]. This bright red salt contains the octahedrally coordinated [Fe(CN)6]3− ion. It is soluble in water and its solution shows some green-yellow fluorescence.
Potassium ferricyanide is manufactured by passing chlorine through a solution of potassium ferrocyanide. Potassium ferricyanide separates from the solution:
Like other metal cyanides, solid potassium ferricyanide has a complicated polymeric structure. The polymer consists of octahedral [Fe(CN)6]3− centers crosslinked with K+ ions that are bound to the CN ligands. The K+---NCFe linkages break when the solid is dissolved in water.
In the 19th century, it was used for reading palimpsests and old manuscripts.
The compound has widespread use in blueprint drawing and in photography (Cyanotype process). Several photographic print toning processes involve the use of potassium ferricyanide. Potassium ferricyanide is used as an oxidizing agent to remove silver from negatives and positives, a process called dot etching. In color photography, potassium ferricyanide is used to reduce the size of color dots without reducing their number, as a kind of manual color correction. It is also used in black-and-white photography with sodium thiosulfate (hypo) to reduce the density of a negative or gelatin silver print where the mixture is known as Farmer's reducer; this can help offset problems from overexposure of the negative, or brighten the highlights in the print.
...
Wikipedia
