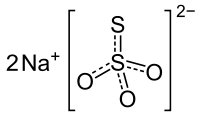
Sodium thiosulfate
 |
|
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Sodium thiosulfate
|
|
| Other names
Sodium hyposulfite
Hyposulphite of soda |
|
| Identifiers | |
|
7772-98-7 10102-17-7 (pentahydrate) |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:132112 |
| ChEMBL |
ChEMBL2096650 (pentahydrate) |
| ChemSpider |
22885 |
| ECHA InfoCard | 100.028.970 |
| E number | E539 (acidity regulators, ...) |
| PubChem | 24477 |
| RTECS number | XN6476000 |
| UNII |
L0IYT1O31N |
|
|
|
|
| Properties | |
| Na2S2O3 | |
| Molar mass | 158.11 g/mol (anhydrous) 248.18 g/mol (pentahydrate) |
| Appearance | White crystals |
| Odor | Odorless |
| Density | 1.667 g/cm3 |
| Melting point | 48.3 °C (118.9 °F; 321.4 K) (pentahydrate) |
| Boiling point | 100 °C (212 °F; 373 K) (pentahydrate, - 5H2O decomposition) |
| 70.1 g/100 mL (20 °C) 231 g/100 mL (100 °C) |
|
| Solubility | negligible in alcohol |
|
Refractive index (nD)
|
1.489 |
| Structure | |
| monoclinic | |
| Hazards | |
| Safety data sheet | External MSDS |
| R-phrases | R35 |
| NFPA 704 | |
| Flash point | Non-flammable |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Sodium thiosulfate (Na2S2O3), also spelled sodium thiosulphate, is a chemical and medication. As a medication it is used to treat cyanide poisoning and pityriasis versicolor.
It is an inorganic compound that is typically available as the pentahydrate, Na2S2O3·5H2O. The solid is an efflorescent (loses water readily) crystalline substance that dissolves well in water. It is also called sodium hyposulfite or “hypo”.
It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system.
In analytical chemistry, the most important use comes because the thiosulfate anion reacts stoichiometrically with iodine in aqueous solution, reducing it to iodide as it is oxidized to tetrathionate:
Due to the quantitative nature of this reaction, as well as because Na2S2O3·5H2O has an excellent shelf-life, it is used as a titrant in iodometry. Na2S2O3·5H2O is also a component of iodine clock experiments.
This particular use can be set up to measure the oxygen content of water through a long series of reactions in the Winkler test for dissolved oxygen. It is also used in estimating volumetrically the concentrations of certain compounds in solution (hydrogen peroxide, for instance) and in estimating the chlorine content in commercial bleaching powder and water.
...
Wikipedia

