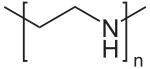
Poly(ethylene imine)
 |
|
| Names | |
|---|---|
|
IUPAC name
Poly(iminoethylene)
|
|
| Other names
Polyaziridine, Poly[imino(1,2-ethanediyl)]
|
|
| Identifiers | |
| ChemSpider |
|
| ECHA InfoCard | 100.123.818 |
| Properties | |
| (C2H5N)n, linear form | |
| Molar mass | 43.04 (repeat unit), mass of polymer variable |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Polyethylenimine (PEI) or polyaziridine is a polymer with repeating unit composed of the amine group and two carbon aliphatic CH2CH2 spacer. Linear polyethyleneimines contain all secondary amines, in contrast to branched PEIs which contain primary, secondary and tertiary amino groups. Totally branched, dendrimeric forms were also reported. PEI is produced on industrial scale and finds many applications usually derived from its polycationic character.
The linear PEIs are solids at room temperature while branched PEIs are liquids at all molecular weights. Linear polyethyleneimines are soluble in hot water, at low pH, in methanol, ethanol, or chloroform. They are insoluble in cold water, benzene, ethyl ether, and acetone. They have a melting point of 73–75 °C. They can be stored at room temperature.
Branched PEI can be synthesized by the ring opening polymerization of aziridine. Depending on the reaction conditions different degree of branching can be achieved. Linear PEI is available by post-modification of other polymers like poly(2-oxazolines) or N-substituted polyaziridines. Linear PEI was synthesised by the hydrolysis of poly(2-ethyl-2-oxazoline) and sold as jetPEI. The current generation in-vivo-jetPEI uses bespoke poly(2-ethyl-2-oxazoline) polymers as precursors.
Polyethyleneimine finds many applications in products like: detergents, adhesives, water treatment agents and cosmetics. Thanks to its ability to modify the surface of cellulose fibres, PEI is employed as a wet-strength agent in the paper-making process. It is also used as flocculating agent with silica sols and as a chelating agent with the ability to complex metal ions such as zinc and zirconium. There are also other highly specialized PEI applications:
PEI has a number of uses in laboratory biology, especially tissue culture, but is also toxic to cells if used in excess. Toxicity is by two different mechanisms, the disruption of the cell membrane leading to necrotic cell death (immediate) and disruption of the mitochondrial membrane after internalisation leading to apoptosis (delayed).
...
Wikipedia
