Pinite
 |
|
| Names | |
|---|---|
|
IUPAC name
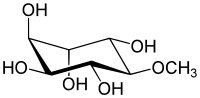
(1S,2S,4S,5R)-6-methoxycyclohexane-1,2,3,4,5-pentol
|
|
| Other names
3-O-Methyl-D-chiro-inositol
D-(+)-chiro-Inositol D-Pinitol Inzitol D-(+)-Pinitol (+)-Pinitol Sennitol Pinnitol (+/-)pinitol |
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
|
PubChem CID
|
|
| UNII | |
|
|
|
|
| Properties | |
| C7H14O6 | |
| Molar mass | 194.18 g/mol |
| Melting point | 179 to 185 °C (354 to 365 °F; 452 to 458 K) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Pinitol is a cyclitol, a cyclic polyol. It is a known anti-diabetic agent isolated from Sutherlandia frutescens leaves.Gall plant tannins can be differentiated by their content of pinitol. It was first identified in the sugar pine (Pinus lambertiana). It is also found in other plants, such as in the pods of the carob tree.
Certain variants of the bacteria Pseudomonas putida have been used in organic synthesis, the first example being the oxidation of benzene, employed by Steven Ley in the synthesis of (+/-)pinitol.
Ciceritol is a pinitol digalactoside that can be isolated from seeds of chickpea, lentil and white lupin.
A cyclitol derivative can be found in the marine sponge Petrosia sp.
...
Wikipedia
