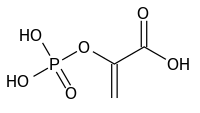
Phosphoenol pyruvate
 |
|
| Names | |
|---|---|
|
IUPAC name
2-(phosphonooxy)acrylic acid
|
|
| Other names
Phosphoenolpyruvic acid, PEP
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.004.830 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C3H5O6P | |
| Molar mass | 168.042 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Phosphoenolpyruvic acid (PEP), or phosphoenolpyruvate (2-phosphoenolpyruvate) as the anion, is an important chemical compound in biochemistry. It has the highest-energy phosphate bond found (-61.9 kJ/mol) in living organisms, and is involved in glycolysis and gluconeogenesis. In plants, it is also involved in the biosynthesis of various aromatic compounds, and in carbon fixation; in bacteria, it is also used as the source of energy for the phosphotransferase system.
PEP is formed by the action of the enzyme enolase on 2-phosphoglyceric acid. Metabolism of PEP to pyruvic acid by pyruvate kinase (PK) generates 1 molecule of adenosine triphosphate (ATP) via substrate-level phosphorylation. ATP is one of the major currencies of chemical energy within cells.
Compound C00631 at KEGG Pathway Database. Enzyme 4.2.1.11 at KEGG Pathway Database. Compound C00074 at KEGG Pathway Database. Enzyme 2.7.1.40 at KEGG Pathway Database. Compound C00022 at KEGG Pathway Database.
...
Wikipedia
