Phenylbutyrate
 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Consumer Drug Information |
| License data |
|
| Pregnancy category |
|
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic and renal to phenylacetic acid |
| Biological half-life | 0.8 hours (phenylbutyrate), 1.15-1.29 hours (phenylacetate) |
| Excretion | Urine (80-100% as phenylacetylglutamine) |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.130.318 |
| Chemical and physical data | |
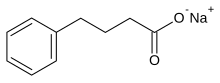
| Formula | C10H11NaO2 |
| Molar mass | 186.2 g/mol |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Sodium phenylbutyrate is a salt of an aromatic fatty acid, 4-phenylbutyrate (4-PBA) or 4-phenylbutyric acid. The compound is used to treat urea cycle disorders, because its metabolites offer an alternative pathway to the urea cycle to allow excretion of excess nitrogen. It is an orphan drug, marketed by Ucyclyd Pharma under the trade name Buphenyl, by Swedish Orphan International (Sweden) as Ammonaps, and by Fyrlklövern Scandinavia as triButyrate.
Sodium phenylbutyrate is also a histone deacetylase inhibitor and chemical chaperone, leading respectively to research into its use as an anti-cancer agent and in protein misfolding diseases such as cystic fibrosis.
Sodium phenylbutyrate is a sodium salt of an aromatic fatty acid, made up of an aromatic ring and butyric acid. The chemical name for sodium phenylbutyrate is 4-phenylbutyric acid, sodium salt. It forms water-soluble off-white crystals.
Sodium phenylbutyrate is taken orally or by nasogastric intubation as a tablet or powder, and tastes very salty and bitter. It treats urea cycle disorders, genetic diseases in which nitrogen waste builds up in the blood plasma as ammonia glutamine (a state called hyperammonemia) due to deficiences in the enzymes carbamoyl phosphate synthetase I, ornithine transcarbamylase, or argininosuccinic acid synthetase. Uncontrolled, this causes mental retardation and early death. Sodium phenylbutyrate metabolites allows the kidneys to excrete excess nitrogen in place of urea, and coupled with dialysis, amino acid supplements and a protein-restricted diet, children born with urea cycle disorders can usually survive beyond 12 months. Patients may need treatment for all their life. The treatment was introduced by researchers in the 1990s, and approved by the FDA on 13 May 1996.
...
Wikipedia
