Perjeta
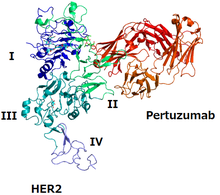
The structure of HER2 and pertuzumab
|
|
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Humanized (from mouse) |
| Target | HER2 |
| Clinical data | |
| Trade names | Perjeta |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
Intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| ChEMBL | |
|
|
|
Pertuzumab (also called 2C4, trade name Perjeta) is a monoclonal antibody used in combination with trastuzumab and docetaxel for the treatment of metastatic HER2-positive breast cancer; it also used in the same combination as a neoadjuvant in early HER2-positive breast cancer.
Side effects in more than half the people taking it include diarrhea, hair loss, and loss of neutrophils; more than 10% experience loss of red blood cells, hypersensitivity or allergic reaction, infusion reactions, decreased appetite, insomnia, distortions in the sense of taste, inflammation of the mouth or lips, constipation, rashes, nail disease, and muscle pain. Women who are pregnant or planning on getting pregnant should not take it, it was not studied in people with certain heart conditions and should be used in caution in such people, and it should not be used with an anthracycline. It is unknown if pertuzumab interacts with doxorubicin.
It is the first-in-class of a kind of drug called a "HER dimerization inhibitor" — it inhibits the dimerization of HER2 with other HER receptors, which prevents them from signalling in ways that promote cell growth and proliferation.
It was discovered and developed by Genentech, a subsidiary of Roche, and was first approved in 2012.
Pertuzumab is administered as an intravenous infusion in combination with trastuzumab and docetaxel as a first line treatment for HER2-positive metastatic breast cancer. It is also used in the same combination as a neoadjuvant (given to reduce the size of a tumor, prior to surgery or radiation) for HER2-positive early breast cancer; as of 2016 this use had not been shown to increase survival.
...
Wikipedia
