Pepcid
 |
|
 |
|
| Clinical data | |
|---|---|
| Pronunciation | /fəˈmɒtɪdiːn/ |
| Trade names | Pepcid, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a687011 |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
Oral (tablets), Intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 40–45% (oral) |
| Protein binding | 15–20% |
| Metabolism | hepatic |
| Biological half-life | 2.5–3.5 hours |
| Excretion | Renal (25-30% unchanged [Oral]) |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.116.793 |
| Chemical and physical data | |
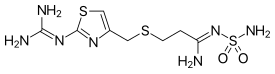
| Formula | C8H15N7O2S3 |
| Molar mass | 337.449 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Famotidine, sold under the trade name Pepcid among others, is a histamine H2 receptor antagonist that inhibits stomach acid production. It is commonly used in the treatment of peptic ulcer disease and gastroesophageal reflux disease.
Unlike cimetidine, the first H2 antagonist, famotidine has no effect on the enzyme system, and does not appear to interact with other drugs.
It was discovered in 1979.
Famotidine is also given to dogs and cats with acid reflux. Famotidine has been used in combination with an H1 antagonist to treat and prevent urticaria caused by an acute allergic reaction.
Certain preparations of famotidine are available over the counter (OTC) in various countries. In the United States and Canada, 10 mg and 20 mg tablets, sometimes in combination with an antacid, are available OTC. Larger doses still require a medical prescription.
Formulations of famotidine in combination with ibuprofen were marketed by Horizon Pharma under the trade name Duexis.
Side effects associated with famotidine use include headache, dizziness, and constipation or diarrhea.
Famotidine was developed by Yamanouchi Pharmaceutical Co. It was licensed in the mid-1980s by Merck & Co. and is marketed by a joint venture between Merck and Johnson & Johnson. The imidazole ring of cimetidine was replaced with a 2-guanidinothiazole ring. Famotidine proved to be 9 times more potent than ranitidine, and 32 times more potent than cimetidine.
...
Wikipedia
