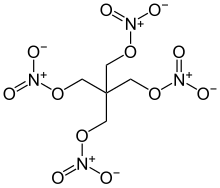
Pentrite
 |
|
 |
|
 |
|
| Names | |
|---|---|
|
Preferred IUPAC name
2,2-Bis[(nitrooxy)methyl]propane-1,3-diyl dinitrate
|
|
| Other names
[3-Nitrooxy-2,2-bis(nitrooxymethyl)propyl] nitrate
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.987 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C5H8N4O12 | |
| Molar mass | 316.137 |
| Appearance | White crystalline solid |
| Density | 1.77 g/cm3 at 20 °C |
| Melting point | 141.3 °C (286.3 °F; 414.4 K) |
| Boiling point | 180 °C (356 °F; 453 K) (decomposes above 150 °C (302 °F)) |
| Explosive data | |
| Shock sensitivity | Medium |
| Friction sensitivity | Medium |
| Detonation velocity | 8400 m/s (density 1.7 g/cm3) |
| RE factor | 1.66 |
| Hazards | |
| GHS pictograms |
  
|
| GHS signal word | Danger |
| H201, H302, H316, H370, H373, H241 | |
| P210, P250, P261, P264, P301+312, P372, P401, P501, P370+380 | |
| NFPA 704 | |
| 190 °C (374 °F; 463 K) | |
| Pharmacology | |
| C01DA05 (WHO) | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Pentaerythritol tetranitrate (PETN), also known as PENT, PENTA, TEN, corpent, penthrite (or—rarely and primarily in German—as nitropenta), is the nitrate ester of pentaerythritol, and is structurally very similar to nitroglycerin. Penta refers to the five carbon atoms of the neopentane skeleton.
PETN is one of the most powerful explosive materials known, with a relative effectiveness factor of 1.66. When mixed with a plasticizer, PETN forms a plastic explosive. Along with RDX it is the main ingredient of Semtex.
PETN is also used as a vasodilator drug to treat certain heart conditions, such as for management of angina.
Pentaerythritol tetranitrate was first prepared and patented in 1894 by the explosives manufacturer Rheinisch-Westfälische Sprengstoff A.G. of Cologne, Germany. The production of PETN started in 1912, when the improved method of production was patented by the German government. PETN was used by the German Military in World War I. It was also used in the MG FF/M autocannons and many other weapon systems of the Luftwaffe in World War II, specifically in the high explosive "Minengeschoß" shell.
PETN is practically insoluble in water (0.01 g/100 ml at 50 °C), weakly soluble in common nonpolar solvents such as aliphatic hydrocarbons (like gasoline) or tetrachloromethane, but soluble in some other organic solvents, particularly in acetone (about 15 g/100 g of the solution at 20 °C, 55 g/100 g at 60 °C) and dimethylformamide (40 g/100 g of the solution at 40 °C, 70 g/100 g at 70 °C). PETN forms eutectic mixtures with some liquid or molten aromatic nitro compounds, e.g. trinitrotoluene (TNT) or tetryl. Due to its highly symmetrical structure, PETN is resistant to attack by many chemical reagents; it does not hydrolyze in water at room temperature or in weaker alkaline aqueous solutions. Water at 100 °C or above causes hydrolysis to dinitrate; presence of 0.1% nitric acid accelerates the reaction.
...
Wikipedia

