Pancuronium bromide
 |
|
 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration |
Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | NA |
| Protein binding | 77 to 91% |
| Metabolism | Hepatic |
| Biological half-life | 1.5 to 2.7 hours |
| Excretion | Renal and biliary |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.035.923 |
| Chemical and physical data | |
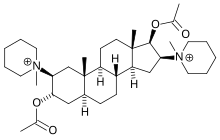
| Formula | C35H60N2O4 |
| Molar mass | 572.861 g/mol |
|
|
|
|
|
Pancuronium (trademarked as Pavulon) is a muscle relaxant with various medical uses. It is used in euthanasia and is the second of three drugs administered during most lethal injections in the United States.
Pancuronium is a typical non-depolarizing curare-mimetic muscle relaxant. It competitively inhibits the nicotinic acetylcholine receptor at the neuromuscular junction by blocking the binding of acetylcholine. It has slight vagolytic activity, causing an increase in heart rate, but no ganglioplegic (i.e., blocking ganglions) activity. It is a very potent muscle relaxant drug, with an ED95 (i.e., the dose that causes 95% depression of muscle twitch response) of only 60 µg/kg body weight. Onset of action is relatively slow compared to other similar drugs, in part due to its low dose - an intubating dose takes 3–6 minutes for full effect. Clinical effects (muscle activity lower than 25% of physiological) last for about 100 minutes. The time needed for full (over 90% muscle activity) recovery after single administration is about 120–180 minutes in healthy adults. The effects of pancuronium can be at least partially reversed by anticholinesterasics, such as neostigmine, pyridostigmine, and edrophonium.
Pancuronium is designed to mimic the action of two molecules of acetylcholine with the quaternary nitrogen atoms spaced rigidly apart by the steroid rings at a distance of ten atoms (interonium distance). Decamethonium and suxamethonium also have this same interonium distance.
...
Wikipedia
