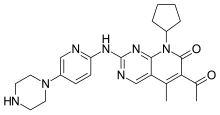
Palbociclib
 |
|
| Clinical data | |
|---|---|
| Trade names | Ibrance |
| AHFS/Drugs.com | ibrance |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
|
|
| Synonyms | PD-0332991 |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| ECHA InfoCard | 100.238.221 |
| Chemical and physical data | |
| Formula | C24H29N7O2 |
| Molar mass | 447.533 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Palbociclib (codenamed PD-0332991, trade name Ibrance) is a drug for the treatment of ER-positive and HER2-negative breast cancer developed by Pfizer. It is a selective inhibitor of the cyclin-dependent kinases CDK4 and CDK6.
It is a selective inhibitor of the cyclin-dependent kinases CDK4 and CDK6.
The drug was reviewed and approved under the Food and Drug Administration’s (FDA) accelerated Priority Review and Breakthrough Therapy designation programs on February 3, 2015 as a treatment (in combination with letrozole) for patients with estrogen receptor positive advanced breast cancer. This was an accelerated approval.
A potentially confirmatory phase 3 trial, PALOMA-2, was fully enrolled by Feb 2015. The PALOMA-2 trial reported positive results in April 2016. The results of PALOMA-2 trial was published in November 2016 which showed significantly longer progression free survival in patients on palociclib in combination with letrozole compared to patients on letrozole alone. However addition of palciclib caused higher rates of myelotoxic events in the study.
The PALOMA-3 trial announced in April 2015 that the addition of palbociclib was superior to fulvestrant alone for progression-free survival.
...
Wikipedia
