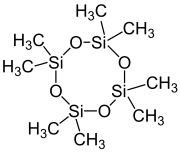
Octamethylcyclotetrasiloxane
 |
|
 |
|
| Names | |
|---|---|
| Other names
D4, OMCTS
|
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.008.307 |
| EC Number | 209-136-7 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C8H24O4Si4 | |
| Molar mass | 296.62 |
| Density | 0.956 g/mL |
| Melting point | 17–18 °C (63–64 °F; 290–291 K) |
| Boiling point | 175–176 °C (347–349 °F; 448–449 K) |
| 56.2±2.5 ppb (23 °C) | |
| Vapor pressure | 124.5±6.2 Pa (25 °C) |
| Related compounds | |
|
Related compounds
|
Disiloxane Tetramethylsilane |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
Tetramethylsilane
Dimethyl ether
Bis(trimethylsilyl)amine Tetrakis(trimethylsilyloxy)silane
Octamethylcyclotetrasiloxane, also called D4, is an organosilicon compound with the formula [(CH3)2SiO]4. lt is a colorless viscous liquid. It is a common cyclomethicone. Like other cyclomethicones, it is slightly volatile. It has attracted scrutiny because it is pervasive in the environment.
Commercially, D4 is produced by cracking polysiloxanes. The silicone polymer equilibrates in the presence of a strong base to give the tetramer
The pentamer decamethylcyclopentasiloxane is also generated. These two cyclic species are separated from the polymer by distillation.
It is among the most important of all the cyclic siloxanes, with a global production volume of 136·106 kilograms in 1993.
As the smallest stable cyclic siloxane, D4 is one of the most abundant siloxanes in the environment, e.g. in landfill gases.
D5 and D4 have attracted attention because they are pervasive. Although never acutely toxic, one report suggests cyclic siloxanes can be detected in some species of aquatic life. However, other scientific reviews have determined that "Siloxane D5 does not pose a danger to the environment."
...
Wikipedia
