Disiloxane
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Disiloxane
|
|
| Other names
Disilyl ether
Disilyl oxide |
|
| Identifiers | |
|
3D model (Jmol)
|
|
| Abbreviations | DS DSE |
| ChEBI | |
| ChemSpider | |
| 1206 | |
| MeSH | Disiloxane |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| H6OSi2 | |
| Molar mass | 78.22 g·mol−1 |
| Appearance | Colorless gas |
| Melting point | −144 °C (−227 °F; 129 K) |
| Boiling point | −15.2 °C (4.6 °F; 257.9 K) |
| 0.24 D | |
| Structure | |
| Orthorhombic | |
| Pmm2 | |
| Bent | |
| Hazards | |
| Safety data sheet | See: data page |
| NFPA 704 | |
| Related compounds | |
|
Related compounds
|
Dimethyl ether |
| Supplementary data page | |
|
Refractive index (n), Dielectric constant (εr), etc. |
|
|
Thermodynamic
data |
Phase behaviour solid–liquid–gas |
| UV, IR, NMR, MS | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
Disilyl oxide
Hexahydrodisiloxane
Perhydrodisiloxane
Silyl ether
DSE
DSO
Disilane
Silane
Silanol
Trisilane
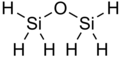
Disiloxane /ˈdaɪˈsaɪlɒkseɪn/ is a chemical compound with the chemical formula Si
2H
6O. It is the simplest siloxane, or oxasilane.
Disiloxane is a molecule with six equivalent Si-H bonds and two equivalent Si-O bonds.
At room temperature and standard pressure, disiloxane is a colourless, pungent gas. Disiloxane has a boiling point of −15 °C (5 °F) at a pressure of one atmosphere.
Today, DSO is primarily produced by converting silane or silicon via gasification to a mixture of silicon monoxide, and hydrogen. This mixture is then converted into DSO in the presence of a catalyst. As described, this is a one-step (direct synthesis) process that permits both silanol synthesis and dehydration in the same process unit, with no silanol isolation and purification. Disiloxane reacts at low temperatures with aluminium halides to give the corresponding silyl and silylene halides and monosilane. Disiloxane is generally considered to be stable in water. It is more soluble than dimethyl ether. It hydrolyses very slowly:
...
Wikipedia

