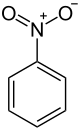
Nitrobenzene
|
|
|||
 |
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
Nitrobenzene
|
|||
| Other names
Nitrobenzol
Oil of mirbane |
|||
| Identifiers | |||
|
98-95-3 |
|||
| 3D model (Jmol) | Interactive image | ||
| ChEBI |
CHEBI:27798 |
||
| ChEMBL |
ChEMBL15750 |
||
| ChemSpider |
7138 |
||
| ECHA InfoCard | 100.002.469 | ||
| KEGG |
C06813 |
||
| PubChem | 7416 | ||
| RTECS number | DA6475000 | ||
| UNII |
E57JCN6SSY |
||
|
|||
|
|||
| Properties | |||
| C6H5NO2 | |||
| Molar mass | 123.06 g/mol | ||
| Appearance | yellowish, oily liquid | ||
| Odor | pungent, like paste shoe polish | ||
| Density | 1.199 g/cm3 | ||
| Melting point | 5.7 °C (42.3 °F; 278.8 K) | ||
| Boiling point | 210.9 °C (411.6 °F; 484.0 K) | ||
| 0.19 g/100 ml at 20 °C | |||
| Vapor pressure | 0.3 mmHg (25°C) | ||
| -61.80·10−6 cm3/mol | |||
| Hazards | |||
|
EU classification (DSD)
|
|
||
| R-phrases |
R10, R23/24/25, R40, R48/23/24, R51/53, R62 |
||
| S-phrases |
(S1/2), S28, S36/37, S45, S61 |
||
| NFPA 704 | |||
| Flash point | 88 °C (190 °F; 361 K) | ||
| 480 °C (896 °F; 753 K) | |||
| Explosive limits | 1.8%-? | ||
| Lethal dose or concentration (LD, LC): | |||
|
LD50 (median dose)
|
780 mg/kg (rat, oral) 600 mg/kg (rat, oral) 590 mg/kg (mouse, oral) |
||
|
LDLo (lowest published)
|
750 mg/kg (dog, oral) | ||
| US health exposure limits (NIOSH): | |||
|
PEL (Permissible)
|
TWA 1 ppm (5 mg/m3) [skin] | ||
|
REL (Recommended)
|
TWA 1 ppm (5 mg/m3) [skin] | ||
|
IDLH (Immediate danger)
|
200 ppm | ||
| Related compounds | |||
|
Related compounds
|
Aniline Benzenediazonium chloride Nitrosobenzene |
||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Nitrobenzene is an organic compound with the chemical formula C6H5NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale from benzene as a precursor to aniline. In the laboratory, it is occasionally used as a solvent, especially for electrophilic reagents.
Nitrobenzene is prepared by nitration of benzene with a mixture of concentrated sulfuric acid, water, and nitric acid. This mixture is sometimes called "mixed acid." The production of nitrobenzene is one of the most dangerous processes conducted in the chemical industry because of the exothermicity of the reaction (ΔH = −117 kJ/mol).
World capacity for nitrobenzene in 1985 was about 1.7×106tonnes.
The nitration process involves formation of the nitronium ion (NO2+), followed by an electrophilic aromatic substitution reaction of it with benzene. The nitronium ion is generated in situ by the reaction of nitric acid and an acidic dehydration agent, typically sulfuric acid:
Approximately 97% of nitrobenzene is consumed in the production of aniline, which is a precursor to rubber chemicals, pesticides, dyes (particularly azo dyes), explosives, and pharmaceuticals.
...
Wikipedia