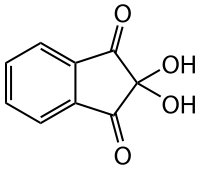
Ninhydrin
 |
|
 |
|
 |
|
| Names | |
|---|---|
|
Preferred IUPAC name
2,2-Dihydroxy-1H-indene-1,3(2H)-dione
|
|
| Other names
2,2-Dihydroxyindane-1,3-dione
1,2,3-Indantrione hydrate |
|
| Identifiers | |
|
485-47-2 |
|
| 3D model (Jmol) | Interactive image |
| ChEMBL |
ChEMBL1221925 |
| ChemSpider |
9819 |
| ECHA InfoCard | 100.006.926 |
| PubChem | 10236 |
|
|
|
|
| Properties | |
| C9H6O4 | |
| Molar mass | 178.14 g·mol−1 |
| Appearance | White solid |
| Density | 0.862 g/cm3 |
| Melting point | 250 °C (482 °F; 523 K) (decomposes) |
| 20 g L−1 | |
| Hazards | |
| Safety data sheet | External MSDS |
| R-phrases | R22, R36, R37, R38 |
| S-phrases | S26, S28, S36 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
 |
|
| Names | |
|---|---|
|
IUPAC name
Indane-1,2,3-trione
|
|
| Other names
Indanetrione
|
|
| Identifiers | |
| 938-24-9 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 63492 |
| ECHA InfoCard | 100.006.926 |
| PubChem | 70309 |
|
|
| Properties | |
| C9H4O3 | |
| Molar mass | 160.13 g·mol−1 |
| Appearance | white powder |
| Density | 1.482 g/cm3 |
| Boiling point | 338.4 °C (641.1 °F; 611.5 K) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
Ninhydrin (2,2-dihydroxyindane-1,3-dione) is a chemical used to detect ammonia or primary and secondary amines. When reacting with these free amines, a deep blue or purple color known as Ruhemann's purple is produced. Ninhydrin is most commonly used to detect fingerprints, as the terminal amines of lysine residues in peptides and proteins sloughed off in fingerprints react with ninhydrin. It is a white solid which is soluble in ethanol and acetone at room temperature. Ninhydrin can be considered as the hydrate of indane-1,2,3-trione.
Ninhydrin was discovered in 1910 by the German-English chemist Siegfried Ruhemann (1859–1943). In the same year, Ruhemann observed ninhydrin's reaction with amino acids. In 1954, Swedish investigators Oden and von Hofsten proposed that ninhydrin could be used to develop latent fingerprints.
Ninhydrin can also be used to monitor deprotection in solid phase peptide synthesis (Kaiser Test). The chain is linked via its C-terminus to the solid support, with the N-terminus extending off it. When that nitrogen is deprotected, a ninhydrin test yields blue. Amino-acid residues are attached with their N-terminus protected, so if the next residue has been successfully coupled onto the chain, the test gives a colorless or yellow result.
Ninhydrin is also used in amino acid analysis of proteins. Most of the amino acids, except proline, are hydrolyzed and react with ninhydrin. Also, certain amino acid chains are degraded. Therefore, separate analysis is required for identifying such amino acids that either react differently or do not react at all with ninhydrin. The rest of the amino acids are then quantified colorimetrically after separation by chromatography.
A solution suspected of containing the ammonium ion can be tested by ninhydrin by dotting it onto a solid support (such as silica gel); treatment with ninhydrin should result in a dramatic purple color if the solution contains this species. In the analysis of a chemical reaction by thin layer chromatography (TLC), the reagent can also be used (usually 0.2% solution in either n-butanol or in ethanol). It will detect, on the TLC plate, virtually all amines, carbamates and also, after vigorous heating, amides.
...
Wikipedia
