Nelfinavir
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Viracept |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a697034 |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Uncertain; increases when taking with food |
| Protein binding | >98% |
| Metabolism | Hepatic by including CYP3A4 and CYP2C19 |
| Biological half-life | 3.5–5 hours |
| Excretion | feces (87%), urine (1–2%) |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| Chemical and physical data | |
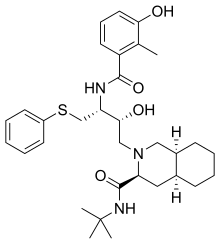
| Formula | C32H45N3O4S |
| Molar mass | 567.784 g/mol |
| 3D model (Jmol) | |
| Melting point | 349.94 °C (661.89 °F) |
|
|
|
|
|
|
|
Nelfinavir (brand name Viracept) is an antiretroviral drug used in the treatment of the human immunodeficiency virus (HIV). Nelfinavir belongs to the class of drugs known as protease inhibitors (PIs) and like other PIs is almost always used in combination with other antiretroviral drugs.
Nelfinavir mesylate (Viracept, formerly AG1343) is a potent and orally bioavailable human immunodeficiency virus HIV-1 protease inhibitor (Ki=2nM) and is widely prescribed in combination with HIV reverse transcriptase inhibitors for the treatment of HIV infection.
Nelfinavir was developed by Agouron Pharmaceuticals as part of a joint venture with Eli Lilly and Company. Agouron Pharmaceuticals was acquired by Warner Lambert in 1999 and is now a subsidiary of Pfizer. It is marketed in Europe by Hoffman-La Roche and elsewhere by ViiV Healthcare.
The U.S. Food and Drug Administration (FDA) approved it for therapeutic use on March 14, 1997, making it the twelfth approved antiretroviral. The initial product launched proved to be the largest "biotech launch" in the history of the pharmaceutical industry, achieving first full year sales exceeding $US335M. Agouron's patent on the drug expired in 2014.
On the 6 June 2007, both the Medicines and Healthcare products Regulatory Agency and the European Medicines Agency put out an alert requesting the recall of any of the drug in circulation, because some batches may have been contaminated with potentially cancer-causing chemicals.
Nelfinavir should be taken with food. The bioavailability of Nelfinavir is increased 2.5 to 5 times when taken with food. Taking the drug with food also decreases the risk of diarrhea as a side effect.
Nelfinavir is a protease inhibitor: it inhibits HIV-1 and HIV-2 proteases. HIV protease is an aspartate protease which splits viral protein molecules into smaller fragments, and it is vital to both the replication of the virus within the cell, and also to the release of mature viral particles from an infected cell. Nelfinavir is a competitive inhibitor (2 nM) which is designed to bind tightly and is not cleaved due to the presence of a hydroxyl group as opposed to a keto group in the middle amino acid residue mimic, which would be otherwise S-phenylcysteine. All protease inhibitors bind to the protease, the precise mode of binding determines how the molecule inhibits the protease. The way Nelfinavir binds the enzyme may be sufficiently unique to reduce cross-resistance between it and other PIs. Also, not all PIs inhibit both HIV-1 and HIV-2 proteases.
...
Wikipedia
