Naringenin
 |
|
| Names | |
|---|---|
|
IUPAC name
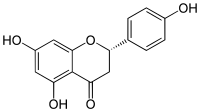
5,7-Dihydroxy-2-(4-hydroxyphenyl)chroman-4-one
|
|
| Other names
Naringetol; Salipurol; Salipurpol; 4',5,7-Trihydroxyflavanone
|
|
| Identifiers | |
|
480-41-1 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:50202 |
| ChEMBL |
ChEMBL9352 |
| ChemSpider |
388383 |
| DrugBank |
DB03467 |
| ECHA InfoCard | 100.006.865 |
| PubChem | 439246 |
| UNII |
HN5425SBF2 |
|
|
|
|
| Properties | |
| C15H12O5 | |
| Molar mass | 272.26 g·mol−1 |
| Melting point | 251 °C (484 °F; 524 K) |
| 475 mg/L | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Naringenin is a flavanone, a type of flavonoid. It is the predominant flavanone in grapefruit.
Naringenin can be found in grapefruit, oranges, tomatoes (skin) and in water mint.
This bioflavonoid is difficult to absorb on oral ingestion. In the best-case scenario, only 15% of ingested naringenin will get absorbed in the human gastrointestinal tract.
The naringenin-7-glucoside form seems less bioavailable than the aglycol form.
Grapefruit juice can provide much higher plasma concentrations of naringenin than orange juice. Also found in grapefruit is the related compound kaempferol, which has a hydroxyl group next to the ketone group.
Naringenin can be absorbed from cooked tomato paste.
Naringenin has been shown to have an inhibitory effect on the human isoform CYP1A2, which can change pharmacokinetics in a human (or orthologous) host of several popular drugs in an adverse manner, even resulting in carcinogens of otherwise harmless substances. The National Research Institute of Chinese Medicine in Taiwan conducted experiments on the effects of the grapefruit flavanones naringin and naringenin on CYP450 enzyme expression. Naringenin proved to be a potent inhibitor of the benzo(a)pyrene metabolizing enzyme benzo(a)pyrene hydroxylase (AHH) in experiments in mice.
Naringenin has also been shown to reduce oxidative damage to DNA in vitro and in animal studies.
Naringenin has also been shown to reduce hepatitis C virus production by infected (liver cells) in cell culture. This seems to be secondary to naringenin's ability to inhibit the secretion of very-low-density lipoprotein by the cells. The antiviral effects of naringenin are currently under clinical investigation.
...
Wikipedia
