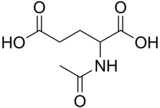
N-Acetylglutamate
 |
|
| Names | |
|---|---|
|
IUPAC name
2-Acetamidopentanedioic acid
|
|
| Other names
Acetylglutamic acid
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| 3DMet | B00147 |
| Abbreviations |
|
| 1727473 S | |
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.024.899 |
| EC Number | 227-388-6 |
| KEGG | |
| MeSH | N-acetylglutamate |
|
PubChem CID
|
|
| RTECS number | LZ9725000 S |
| UNII | |
|
|
|
|
| Properties | |
| C7H11NO5 | |
| Molar mass | 189.17 g·mol−1 |
| Appearance | White crystals |
| Density | 1 g mL−1 |
| Melting point | 191 to 194 °C (376 to 381 °F; 464 to 467 K) |
| 36 g L−1 | |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose)
|
>7 g kg−1(oral, rat) |
| Related compounds | |
|
Related alkanoic acids
|
|
|
Related compounds
|
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
N-Acetylglutamic acid (abbreviated NAcGlu) is biosynthesized from glutamic acid and acetyl-CoA by the enzyme N-acetylglutamate synthase. Arginine is the activator for this reaction.
The reverse reaction, hydrolysis of the acetyl group, is catalyzed by a specific hydrolase.
NAcGlu activates carbamoyl phosphate synthetase in the urea cycle.
...
Wikipedia
