Movantik
 |
|
| Clinical data | |
|---|---|
| Trade names | Movantik, Moventig |
| Synonyms | NKTR-118 |
| AHFS/Drugs.com | movantik |
| License data | |
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | ~4.2% |
| Metabolism | Hepatic (CYP3A) |
| Biological half-life | 6–11 h |
| Excretion | Feces (68%), urine (16%) |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| Chemical and physical data | |
| Formula | C34H53NO11 |
| Molar mass | 651.785 g/mol |
| 3D model (JSmol) | |
|
|
|
|
Naloxegol (INN; PEGylated naloxol; trade names Movantik and Moventig) is a peripherally selective opioid antagonist developed by AstraZeneca, licensed from Nektar Therapeutics, for the treatment of opioid-induced constipation. It was approved in 2014 in adult patients with chronic, non-cancer pain. Doses of 25 mg were found safe and well tolerated for 52 weeks. When given concomitantly with opioid analgesics, naloxegol reduced constipation-related side effects, while maintaining comparable levels of analgesia.
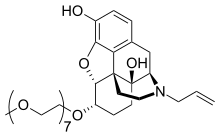
Chemically, naloxegol is a pegylated (polyethylene glycol-modified) derivative of α-naloxol. Specifically, the 6-α-hydroxyl group of α-naloxol is connected via an ether linkage to the free hydroxyl group of a monomethoxy-terminated n=7 oligomer of PEG, shown extending at the lower left of the molecule image at right. The "n=7" defines the number of two-carbon ethylenes, and so the chain length, of the attached PEG chain, and the "monomethoxy" indicates that the terminal hydroxyl group of the PEG is "capped" with a methyl group. The pegylation of the 6-α-hydroxyl side chain of naloxol prevents the drug from crossing the blood-brain barrier (BBB). As such, it can be considered the antithesis of the peripherally-acting opiate loperamide which is utilized as an opiate-targeting anti-diarrheal agent that does not cause traditional opiate side-effects due to its inability to accumulate in the central nervous system in normal subjects.
...
Wikipedia
