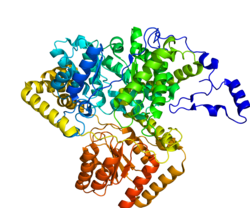
Methylmalonyl-CoA mutase
2XIJ, 2XIQ, 3BIC
4594
17850
ENSG00000146085
ENSMUSG00000023921
P22033
P16332
NM_000255
NM_008650
NP_000246
NP_000246.2
NP_032676.2
NP_032676
Methylmalonyl-CoA mutase (MCM), mitochondrial, also known as methylmalonyl-CoA isomerase, is a protein that in humans is encoded by the MUT gene. This vitamin B12-dependent enzyme catalyzes the isomerization of methylmalonyl-CoA to succinyl-CoA in humans. Mutations in MUT gene may lead to various types of methylmalonic aciduria.
MCM was first identified in rat liver and sheep kidney in 1955. In its latent form, it is 750 amino acids in length. Upon entry to the mitochondria, the 32 amino acid mitochondrial leader sequence at the N-terminus of the protein is cleaved, forming the fully processed monomer. The monomers then associate into homodimers, and bind AdoCbl (one for each monomer active site) to form the final, active holoenzyme form.
...
Wikipedia