Methylcellulose
 |
|
| Names | |
|---|---|
| Other names
Cellulose, methyl ether; methylated cellulose; methylcellulose; E461
|
|
| Identifiers | |
|
9004-67-5 |
|
| ECHA InfoCard | 100.115.188 |
| E number | E461 (thickeners, ...) |
| Properties | |
| variable | |
| Molar mass | variable |
| Pharmacology | |
| A06AC06 (WHO) | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Methyl cellulose (or methylcellulose) is a chemical compound derived from cellulose. It is a hydrophilic white powder in pure form and dissolves in cold (but not in hot) water, forming a clear viscous solution or gel. It is sold under a variety of trade names and is used as a thickener and emulsifier in various food and cosmetic products, and also as a treatment of constipation. Like cellulose, it is not digestible, not toxic, and not an allergen.
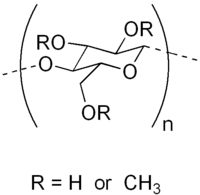
Methyl cellulose does not occur naturally and is synthetically produced by heating cellulose with caustic solution (e.g. a solution of sodium hydroxide) and treating it with methyl chloride. In the substitution reaction that follows, the hydroxyl residues (-OH functional groups) are replaced by methoxide (-OCH3 groups).
Different kinds of methyl cellulose can be prepared depending on the number of hydroxyl groups substituted. Cellulose is a polymer consisting of numerous linked glucose molecules, each of which exposes three hydroxyl groups. The Degree of Substitution (DS) of a given form of methyl cellulose is defined as the average number of substituted hydroxyl groups per glucose. The theoretical maximum is thus a DS of 3.0, however more typical values are 1.3–2.6.
Different methyl cellulose preparations can also differ in the average length of their polymer backbones.
Methyl cellulose has a lower critical solution temperature (LCST) between 40 °C and 50 °C. At temperatures below the LCST, it is readily soluble in water; above the LCST, it is not soluble, which has a paradoxical effect that heating a saturated solution of methyl cellulose will turn it solid, because methyl cellulose will precipitate out. The temperature at which this occurs depends on DS-value, with higher DS-values giving lower solubility and lower precipitation temperatures because the polar hydroxyl groups are masked.
...
Wikipedia
