Lawesson's reagent
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
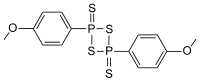
2,4-Bis(4-methoxyphenyl)-1,3,2,4-dithiadiphosphetane-2,4-disulfide
|
|
|
Preferred IUPAC name
2,4-Bis(4-methoxyphenyl)-1,3,2,4-dithiadiphosphetane-2,4-dithione
|
|
| Other names
Lawesson reagent; LR
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.038.944 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C14H14O2P2S4 | |
| Molar mass | 404.45 g·mol−1 |
| Appearance | Slightly yellow powder |
| Melting point | 228–231 °C (442–448 °F; 501–504 K) |
| Insoluble | |
| Hazards | |
|
EU classification (DSD) (outdated)
|
Irritant Harmful (XN) |
| R-phrases (outdated) | R15/29-R20/21/22 |
| S-phrases (outdated) | S7/8-S22-S45 |
| Related compounds | |
|
Related thiation agents
|
Hydrogen sulfide, Phosphorus pentasulfide |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Lawesson's reagent, or LR, is a chemical compound used in organic synthesis as a thiation agent. Lawesson's reagent was first made popular by Sven-Olov Lawesson, who did not, however, invent it. Lawesson's reagent was first made in 1956 during a systematic study of the reactions of arenes with P4S10.
Lawesson's reagent is commercially available. It can also be conveniently prepared in the laboratory by heating a mixture of anisole with phosphorus pentasulfide until the mixture is clear and no more hydrogen sulfide is formed, then recrystallized from toluene or xylene.
As Lawesson's reagent has a strong and unpleasant smell, it is best to prepare the compound within a fume-hood and to treat all glassware used with a decontamination solution before taking the glassware outside the fume-hood. One common and effective method of destroying the foul smelling residues is to use an excess of sodium hypochlorite (chlorine bleach).
Lawesson's reagent has a four membered ring of alternating sulfur and phosphorus atoms. With heating, the central phosphorus/sulfur four-membered ring can open to form two reactive dithiophosphine ylides (R-PS2). Much of the chemistry of Lawessons's reagent is in fact the chemistry of these reactive intermediates.
In general, the more electron rich a carbonyl is, the faster the carbonyl group will be converted into the corresponding thiocarbonyl by Lawesson's reagent.
The chemistry of Lawesson's reagent and related substances has been reviewed by several groups. The main use of Lawesson's reagent is the thionation of carbonyl compounds. For instance, Lawesson's reagent will convert a carbonyl into a thiocarbonyl. Additionally, Lawesson's reagent has been used to thionate enones, esters,lactones,amides, lactams, and quinones.
...
Wikipedia
