Latuda
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Latuda |
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a611016 |
| License data | |
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | N05AE05 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 9–19% (oral) |
| Metabolism | Hepatic (CYP3A4-mediated) |
| Biological half-life | 18 hours |
| Excretion | Faecal (~80%), renal (~9%) |
| Identifiers | |
|
|
| Synonyms | SM-13,496 |
| CAS Number | 367514-87-2 |
| PubChem (CID) | 213046 |
| IUPHAR/BPS | 7461 |
| ChemSpider | 184739 |
| UNII |
22IC88528T |
| ChEBI |
CHEBI:70735 |
| ChEMBL | CHEMBL1237021 |
| ECHA InfoCard | 100.225.187 |
| Chemical and physical data | |
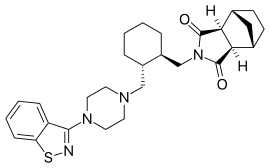
| Formula | C28H36N4O2S |
| Molar mass | 492.68 g/mol |
| 3D model (Jmol) | Interactive image |
| Specific rotation | [α]20D −59° |
| Melting point | 176 to 178 °C (349 to 352 °F) |
| Solubility in water | 45 mg/mL (20 °C) |
|
|
|
|
Lurasidone /lɜːr.ˈræ.sɪ.doʊn/ (trade name Latuda) is an atypical antipsychotic developed by Dainippon Sumitomo Pharma and marketed by Sunovion in the USA. It has been approved for treatment of schizophrenia by the FDA since 2010. In the USA since 2013, it is also approved for the treatment of depressive episodes associated with bipolar I disorder as well as bipolar II disorder in adults when used alone or in combination with lithium, valproate, or lamotrigine.
Lurasidone is FDA approved for the treatment of schizophrenia since 2010 and depressive episodes associated with bipolar I disorder since 2013. It received regulatory approval in the UK in September 2014. In October 2014, NHS Scotland advised use of lurasidone for schizophrenic adults who have not seen improvements with previous antipsychotics due to problems that arise from weight gain or changes in metabolic pathways when taking other medications. It received EMA approval on January 24, 2014. It was launched in Canada for the treatment of schizophrenia in September 2012, Health Canada giving their Summary Basis of Decision (SBD) as favourable on October 15, 2012. European Commission has granted a marketing authorization for once-daily oral lurasidone for the treatment of schizophrenia in adults. It is approved for use in the EU.
...
Wikipedia
