Latanoprost
 |
|
 |
|
| Clinical data | |
|---|---|
| Pronunciation | la-TAN-oh-prost |
| Trade names | Xalatan, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a697003 |
| Pregnancy category |
|
| Routes of administration |
Topical (eye drops) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Activation by ester hydrolysis, deactivation by beta oxidation |
| Onset of action | 3–4 hours |
| Biological half-life | 17 minutes (plasma) |
| Duration of action | ≥ 24 hours |
| Excretion | Mainly via kidney |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.162.178 |
| Chemical and physical data | |
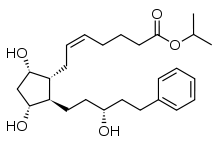
| Formula | C26H40O5 |
| Molar mass | 432.593 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Latanoprost, sold under the brand name Xalatan among others, is a medication used to treat increased pressure inside the eye. This includes ocular hypertension and open angle glaucoma. It is applied as eye drops to the eyes. Onset of effects is usually within four hours, and they last for up to a day.
Common side effects include blurry vision, redness of the eye, itchiness, and darkening of the iris. Latanoprost is in the prostaglandin analogue family of medication. It works by increasing the outflow of aqueous fluid from the eyes through the uveoscleral tract.
Latanoprost approved for medical use in the United States in 1996. It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system. Latanoprost is available as a generic medication. The wholesale cost in the developing world is about 0.69 to 3.79 USD per 2.5 ml bottle. In the United States a month of treatment costs less than 25 USD.
In people with ocular hypertension including open-angle glaucoma (IOP ≥21 mm Hg), treatment with latanoprost reduced IOP levels by 22 to 39% over 1 to 12 months’ treatment. Latanoprost was more effective than timolol 0.5% twice daily in 3 of 4 large (n = 163 to 267) randomised, double-blind trials. Latanoprost demonstrated a stable long-term IOP-lowering effect in 1- or 2-year continuations of these trials, with no sign of diminishing effect during prolonged treatment.
...
Wikipedia
