Kynurenine
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
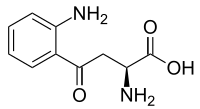
(S)-2-Amino-4-(2-aminophenyl)- 4-oxo-butanoic acid
|
|
| Other names
(S)-Kynurenine
|
|
| Identifiers | |
|
343-65-7 (D/L) 2922-83-0 (L) 13441-51-5 (D) |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:57959 |
| ChEMBL |
ChEMBL498416 |
| ChemSpider |
141580 |
| DrugBank |
DB02070 |
| ECHA InfoCard | 100.005.860 |
| MeSH | Kynurenine |
| PubChem | 846 |
|
|
|
|
| Properties | |
| C10H12N2O3 | |
| Molar mass | 208.22 g·mol−1 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
L-Kynurenine is a metabolite of the amino acid L-tryptophan used in the production of niacin.
Kynurenine is synthesized by the enzyme tryptophan dioxygenase, which is made primarily but not exclusively in the liver, and indoleamine 2,3-dioxygenase, which is made in many tissues in response to immune activation. Kynurenine and its further breakdown products carry out diverse biological functions, including dilating blood vessels during inflammation and regulating the immune response. Some cancers increase kynurenine production, which increases tumor growth.
Evidence suggests that increased kynurenine production may precipitate depressive symptoms associated with interferon treatment for hepatitis C. Cognitive deficits in schizophrenia are associated with imbalances in the enzymes that break down kynurenine. Kynurenine production is increased in Alzheimer's disease and cardiovascular disease where its metabolites are associated with cognitive deficits and depressive symptoms. Kynurenine is also associated with tics.
Kynureninase catabolizes the conversion of kynurenine into anthranilic acid while kynurenine-oxoglutarate transaminase catabolizes its conversion into kynurenic acid. Kynurenine 3-hydroxylase converts kynurenine to 3-hydroxykynurenine.
Dysfunctional states of distinct steps of the kynurenine pathway (e.g. kynurenine, kynurenic acid, quinolinic acid, anthranilic acid, 3 -Hydroxykynurenine) have been described for a number of disorders, e.g.:
Downregulation of kynurenine-3-monooxygenase (KMO) can be caused by genetic polymorphisms, cytokines, or both. KMO deficiency leads to an accumulation of kynurenine and to a shift within the tryptophan metabolic pathway towards kynurenine acid and anthranilic acid. Kynurenine-3-monooxygenase deficiency is associated with disorders of the brain (e.g. schizophrenia, tic disorders) and of the liver.
...
Wikipedia
