Kupffer cells
| Kupffer cell | |
|---|---|

Centrilobular Kupffer cells, with a grey granular cytoplasm, in a resolving liver injury. Liver biopsy. Trichrome stain.
|
|
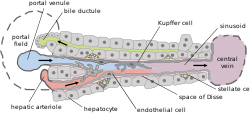
Basic liver structure
|
|
| Details | |
| Identifiers | |
| Latin | macrophagocytus stellatus |
| MeSH | Kupffer+Cells |
| Code | TH H3.04.05.0.00016 |
|
Anatomical terminology
[]
|
|
Kupffer cells, also known as stellate macrophages and Kupffer-Browicz cells, are specialized macrophages located in the liver, lining the walls of the sinusoids that form part of the mononuclear phagocyte system.
The cells were first observed by Karl Wilhelm von Kupffer in 1876. The scientist called them "Sternzellen" (star cells or hepatic stellate cell) but thought, inaccurately, that they were an integral part of the endothelium of the liver blood vessels and that they originated from it. In 1898, after several years of research, Tadeusz Browicz identified them, correctly, as macrophages.
Their development begins in the yolk sac where they differentiate into fetal macrophages. Once they enter the blood stream, they migrate to the fetal liver where they reside. There they complete their differentiation into Kupffer cells.
Red blood cells are broken down by phagocytic action, where the hemoglobin molecule is split. The globin chains are re-utilized, while the iron-containing portion, heme, is further broken down into iron, which is re-utilized, and bilirubin, which is conjugated to glucuronic acid within and secreted into the bile.
Helmy et al. identified a receptor present in Kupffer cells, the complement receptor of the immunoglobulin family (CRIg). Mice without CRIg could not clear complement system-coated pathogens. CRIg is conserved in mice and humans and is a critical component of the innate immune system.
Kupffer cell activation is responsible for early ethanol-induced liver injury, common in chronic alcoholics. Chronic alcoholism and liver injury deal with a two hit system. The second hit is characterized by an activation of the Toll-like receptor 4 (TLR4) and CD14, receptors on the Kupffer cell that internalize endotoxin (lipopolysaccharide or LPS). This activates the transcription of pro-inflammatory cytokines (Tumor necrosis factor-alpha or TNFα) and production of superoxides (a pro-oxidant). TNFα will then enter the stellate cell in the liver, leading to collagen synthesis and fibrosis. Fibrosis will eventually cause cirrhosis, or loss of function of the liver.
...
Wikipedia
