Hemoglobin
|
hemoglobin
(heterotetramer, (αβ)2) |
||
 |
||
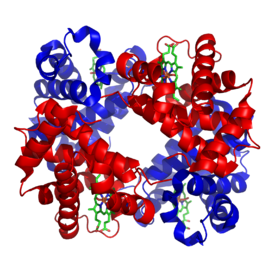
| Structure of human hemoglobin. The proteins α and β subunits are in red and blue, and the iron-containing heme groups in green. From PDB: 1GZX Proteopedia Hemoglobin | ||
| − | ||
| Protein type | metalloprotein, globulin | |
| Function | oxygen-transport | |
| Cofactor(s) | heme (4) | |
| − | ||
| Subunit name |
Gene | Chromosomal locus |
| Hb-α1 | HBA1 | Chr. 16 p13.3 |
| Hb-α2 | HBA2 | Chr. 16 p13.3 |
| Hb-β | HBB | Chr. 11 p15.5 |
Hemoglobin (/ˈhiːməˌɡloʊbᵻn, ˈhɛ-, -moʊ-/); also spelled haemoglobin (United Kingdom spelling) and abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein in the red blood cells of all vertebrates (with the exception of the fish family Channichthyidae) as well as the tissues of some invertebrates. Hemoglobin in the blood carries oxygen from the respiratory organs (lungs or gills) to the rest of the body (i.e. the tissues). There it releases the oxygen to permit aerobic respiration to provide energy to power the functions of the organism in the process called metabolism.
In mammals, the protein makes up about 96% of the red blood cells' dry content (by weight), and around 35% of the total content (including water). Hemoglobin has an oxygen-binding capacity of 1.34 mL O2 per gram, which increases the total blood oxygen capacity seventy-fold compared to dissolved oxygen in blood. The mammalian hemoglobin molecule can bind (carry) up to four oxygen molecules.
...
Wikipedia
