Iodine pentafluoride
|
|
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
Iodine(V) fluoride
|
|||
|
Systematic IUPAC name
Pentafluoro-λ5-iodane
|
|||
| Other names
Iodic fluoride
|
|||
| Identifiers | |||
|
7783-66-6 |
|||
| 3D model (Jmol) | Interactive image | ||
| ChemSpider |
455940 |
||
| ECHA InfoCard | 100.029.108 | ||
| EC Number | 232-019-7 | ||
| PubChem | 522683 | ||
|
|||
|
|||
| Properties | |||
| IF5 | |||
| Molar mass | 221.89 g/mol | ||
| Appearance | colorless or pale yellow liquid | ||
| Density | 3.250 g/cm3 | ||
| Melting point | 9.43 °C (48.97 °F; 282.58 K) | ||
| Boiling point | 97.85 °C (208.13 °F; 371.00 K) | ||
| Reacts | |||
| −58.1·10−6 cm3/mol | |||
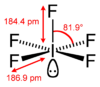
| Structure | |||
|
monoclinic point group C2/c |
|||
| square pyramidal | |||
| square pyramidal | |||
| Hazards | |||
| Main hazards | Toxic, oxidizing, corrosive. | ||
| Safety data sheet | External MSDS | ||
| GHS pictograms |
   
|
||
| GHS signal word | Danger | ||
| H271, H330, H311, H301, H314, H371, H410 | |||
| P202, P232, P304, P310 | |||
| NFPA 704 | |||
| Related compounds | |||
|
Other anions
|
Iodine pentoxide | ||
|
Other cations
|
Bromine pentafluoride | ||
|
Related compounds
|
Iodine monofluoride Iodine trifluoride Iodine heptafluoride |
||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Iodine pentafluoride is an interhalogen compound with chemical formula IF5. It is a fluoride of iodine. It is a colorless or yellow liquid with a density of 3.250 g cm−3. It was first synthesized by Henri Moissan in 1891 by burning solid iodine in fluorine gas. This exothermic reaction is still used to produce iodine pentafluoride, although the reaction conditions have been improved.
Iodine pentafluoride is a strong fluorination agent and is highly oxidative. It reacts vigorously with water forming hydrofluoric acid and with more fluorine forming iodine heptafluoride.
Primary amines react with iodine pentafluoride forming nitriles after hydrolysis with water.
...
Wikipedia