Inosine
 |
|
 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.000.355 |
| Chemical and physical data | |
| Formula | C10H12N4O5 |
| Molar mass | 268.23 g/mol |
| 3D model (Jmol) | |
|
|
|
|
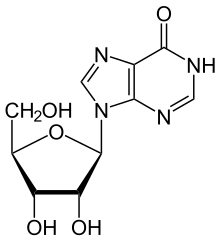
Inosine is a nucleoside that is formed when hypoxanthine is attached to a ribose ring (also known as a ribofuranose) via a β-N9-glycosidic bond.
Inosine is commonly found in tRNAs and is essential for proper translation of the genetic code in wobble base pairs.
Knowledge of inosine metabolism has led to advances in immunotherapy in recent decades. Inosine monophosphate is oxidised by the enzyme inosine monophosphate dehydrogenase, yielding xanthosine monophosphate, a key precursor in purine metabolism. Mycophenolate mofetil is an anti-metabolite, anti-proliferative drug that acts as an inhibitor of inosine monophosphate dehydrogenase. It is used in the treatment of a variety of autoimmune diseases including granulomatosis with polyangiitis because the uptake of purine by actively dividing B cells can exceed 8 times that of normal body cells, and, therefore, this set of white cells (which cannot operate purine salvage pathways) is selectively targeted by the purine deficiency resulting from inherited metabolic diseases (IMD) inhibition.
Adenine is converted to adenosine or inosine monophosphate (IMP), either of which, in turn, is converted into inosine (I), which pairs with adenine (A), cytosine (C), and uracil (U).
Purine nucleoside phosphorylase intraconverts inosine and hypoxanthine.
...
Wikipedia
