Hypertonic saline
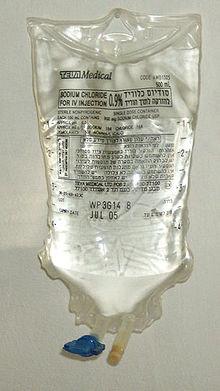
Saline solution for intravenous infusion. The white port at the base of the bag is where additives can be injected. The port with the blue cover is where the bag is spiked with an infusion set.
|
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | FDA Professional Drug Information |
| Routes of administration |
intravenous, topical, subcutaneous |
| ATC code | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| 3D model (Jmol) | |
|
|
|
|
Saline, also known as saline solution, is a mixture of sodium chloride in water and has a number of uses in medicine. Applied to the affected area it is used to clean wounds, help remove contact lenses, and help with dry eyes. By injection into a vein it is used to treat dehydration such as from gastroenteritis and diabetic ketoacidosis. It is also used to dilute other medications to be given by injection.
Large amounts may result in fluid overload, swelling, acidosis, and high blood sodium. In those with long standing low blood sodium excessive use may result in osmotic demyelination syndrome. Saline is in the crystalloid family of medications. It is most commonly used as a sterile 9 g of salt per litre (0.9%) solution, known as normal saline. Higher and lower concentrations may also occasionally be used.
The medical use of saline began around 1831. It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system. The wholesale cost in the developing world is about 0.60 to 4.20 USD per liter of normal saline.
Concentrations vary from low to normal to high. High concentrations are used rarely in medicine but frequently in molecular biology.
...
Wikipedia
