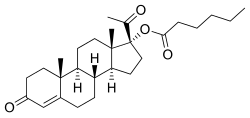
Hydroxyprogesterone caproate
 |
|
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration |
Intramuscular injection |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability |
Oral: Very low (~3% in rats) Intramuscular: 100% (in rats) |
| Biological half-life | Non-pregnant: 7.8 days Singlet: 16 days Twins: 10 days |
| Identifiers | |
|
|
| Synonyms | 17α-Hydroxyprogesterone caproate, 17α-OHPC, 17-Hydroxyprogesterone caproate, 17-OHPC, 17-HPC, 17α-HPC, HPC |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ECHA InfoCard | 100.010.127 |
| Chemical and physical data | |
| Formula | C27H40O4 |
| Molar mass | 428.6041 g/mol |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Hydroxyprogesterone caproate (OHPC) (INN, USAN, JAN) (brand names Delalutin, Proluton, Makena, Prodrox, Hylutin, many others), also known as hydroxyprogesterone hexanoate (BANM), is a steroidal progestin and derivative of 17α-hydroxyprogesterone (17α-OHP) that is related to other 17α-OHP derivatives such as chlormadinone acetate, cyproterone acetate, medroxyprogesterone acetate, and megestrol acetate. It is an ester of 17α-OHP formed from caproic acid (hexanoic acid).
OHPC was previously marketed under the trade name Delalutin by Squibb, which was approved by the United States (U.S.) Food and Drug Administration (FDA) in 1956 and withdrawn from marketing in 1999. It is also sold as Proluton throughout Europe. The U.S. FDA approved Makena from KV Pharmaceutical (previously named as Gestiva) on February 4, 2011 for prevention of preterm delivery in women with a history of preterm delivery, sparking a pricing controversy.
OHPC is used in the treatment of threatened miscarriage, gynecological disorders such as dysmenorrhea, premenstrual syndrome, fibrocystic breast disease, adenosis, and breast pain, and endometrial cancer. It was used widely in the 1950s through the 1970s for these indications, but OHPC more recently has received the most attention in the prevention of preterm birth.
...
Wikipedia
