Megestrol acetate
 |
|
 |
|
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration |
Oral (tablets, suspension) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | poor |
| Protein binding | Majority to albumin (no affinity for SHBG or CBG) |
| Biological half-life | 13–105 hours (mean 34) |
| Identifiers | |
|
|
| Synonyms | BDH-1298, NSC-71423 |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| ECHA InfoCard | 100.008.969 |
| Chemical and physical data | |
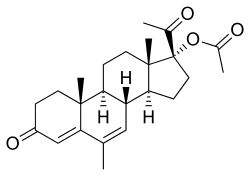
| Formula | C24H32O4 |
| Molar mass | 384.509 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Megestrol acetate (MGA) (INN, USAN, BAN, JAN) (brand names Megace, Megace ES), also known as 17α-acetoxy-6-dehydro-6-methylprogesterone, is a steroidal progestin of the 17α-hydroxyprogesterone group that is used in the treatment of breast and endometrial cancer and as an appetite stimulant. It is the 17α-acetate ester of megestrol, which, in contrast to MGA, was never marketed for clinical use. The term megestrol is often inappropriately used as a synonym for MGA, and when it is used, it almost always refers to MGA rather actually than megestrol.
MGA is used mainly as an appetite stimulant in a variety of conditions and as an antineoplastic agent in the treatment of breast, endometrial, and prostate cancers. When given in relatively high doses, it can substantially increase appetite in most individuals, even those with advanced cancer, and is often used to boost appetite and induce weight gain in patients with cancer or HIV/AIDS-associated cachexia. It is also used as a contraceptive in combination with an estrogen at relatively low doses.
...
Wikipedia
