Hydrocyanic acid
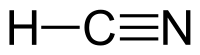 |
|||
|
|
|||
| Names | |||
|---|---|---|---|
IUPAC name
|
|||
Other names
|
|||
| Identifiers | |||
|
74-90-8 |
|||
| 3D model (Jmol) | Interactive image | ||
| 3DMet | B00275 | ||
| ChEBI |
CHEBI:18407 |
||
| ChemSpider |
748 |
||
| ECHA InfoCard | 100.000.747 | ||
| EC Number | 200-821-6 | ||
| KEGG |
C01326 |
||
| MeSH | Hydrogen+Cyanide | ||
| PubChem | 768 | ||
| RTECS number | MW6825000 | ||
| UNII |
2WTB3V159F |
||
| UN number | 1051 | ||
|
|||
|
|||
| Properties | |||
| HCN | |||
| Appearance | Very pale, blue, transparent liquid or colorless gas | ||
| Odor | Oil of bitter almond | ||
| Density | 0.687 g mL−1 | ||
| Melting point | −14 to −12 °C; 7 to 10 °F; 259 to 261 K | ||
| Boiling point | 25.6 to 26.6 °C; 78.0 to 79.8 °F; 298.7 to 299.7 K | ||
| Miscible | |||
| Solubility in ethanol | Miscible | ||
| Vapor pressure | 630 mmHg (20 °C) | ||
|
Henry's law
constant (kH) |
75 μmol Pa−1 kg−1 | ||
| Acidity (pKa) | 9.21 | ||
| Basicity (pKb) | 4.79 | ||
|
Refractive index (nD)
|
1.2675 | ||
| Viscosity | 201 μPa s | ||
| Structure | |||
| C∞v | |||
| Linear | |||
| 2.98 D | |||
| Thermochemistry | |||
| 71.00 kJ K−1 mol−1 (at 27 °C) | |||
|
Std molar
entropy (S |
113.01 J K−1 mol−1 | ||
|
Std enthalpy of
formation (ΔfH |
109.9 kJ mol−1 | ||
|
Std enthalpy of
combustion (ΔcH |
-426.5 kJ mol−1 | ||
| Hazards | |||
| GHS pictograms |
  
|
||
| GHS signal word | DANGER | ||
| H225, H319, H336 | |||
| P210, P261, P305+351+338 | |||
|
EU classification (DSD)
|
|
||
| R-phrases | R12, R26/27/28, R50/53 | ||
| S-phrases | (S1/2), S16, S36/37, S38, S45, S53, S59, S61 | ||
| NFPA 704 | |||
| Flash point | −17.8 °C (0.0 °F; 255.3 K) | ||
| 538 °C (1,000 °F; 811 K) | |||
| Explosive limits | 5.6% – 40.0% | ||
| Lethal dose or concentration (LD, LC): | |||
|
LC50 (median concentration)
|
501 ppm (rat, 5 min) 323 ppm (mouse, 5 min) 275 ppm (rat, 15 min) 170 ppm (rat, 30 min) 160 ppm (rat, 30 min) 323 ppm (rat, 5 min) |
||
|
LCLo (lowest published)
|
200 ppm (mammal, 5 min) 36 ppm (mammal, 2 hr) 107 ppm (human, 10 min) 759 ppm (rabbit, 1 min) 759 ppm (cat, 1 min) 357 ppm (human, 2 min) 179 ppm (human, 1 hr) |
||
| US health exposure limits (NIOSH): | |||
|
PEL (Permissible)
|
TWA 10 ppm (11 mg/m3) [skin] | ||
|
REL (Recommended)
|
ST 4.7 ppm (5 mg/m3) [skin] | ||
|
IDLH (Immediate danger)
|
50 ppm | ||
| Related compounds | |||
|
Related alkanenitriles
|
|||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Hydrogen cyanide (HCN), sometimes called prussic acid, is an organic compound with the chemical formula HCN. It is a colorless, extremely poisonous and flammable liquid that boils slightly above room temperature, at 25.6 °C (78.1 °F). HCN is produced on an industrial scale and is a highly valuable precursor to many chemical compounds ranging from polymers to pharmaceuticals.
Hydrogen cyanide is a linear molecule, with a triple bond between carbon and nitrogen. A minor tautomer of HCN is HNC, hydrogen isocyanide.
Hydrogen cyanide is weakly acidic with a pKa of 9.2. It partially ionizes in water solution to give the cyanide anion, CN−. A solution of hydrogen cyanide in water, represented as HCN, is called hydrocyanic acid. The salts of the cyanide anion are known as cyanides.
HCN has a faint bitter almond-like odor that some people are unable to detect owing to a recessive genetic trait. The volatile compound has been used as inhalation rodenticide and human poison, as well as for killing whales. Cyanide ions interfere with iron-containing respiratory enzymes.
...
Wikipedia


