Hydrazine hydrate
|
|
|||
|
|
|||
 Hydrazine hydrate
|
|||
| Names | |||
|---|---|---|---|
|
Systematic IUPAC name
Hydrazine
|
|||
| Other names
Diamine; Diazane; Tetrahydridodinitrogen (N—N)
|
|||
| Identifiers | |||
|
3D model (Jmol)
|
|||
| 3DMet | B00770 | ||
| 878137 | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.005.560 | ||
| EC Number | 206-114-9 | ||
| 190 | |||
| KEGG | |||
| MeSH | Hydrazine | ||
|
PubChem CID
|
|||
| RTECS number | MU7175000 | ||
| UNII | |||
| UN number | 2029 | ||
|
|||
|
|||
| Properties | |||
| N 2H 4 |
|||
| Molar mass | 32.0452 g mol−1 | ||
| Appearance | Colorless, fuming, oily liquid | ||
| Odor | ammonia-like | ||
| Density | 1.021 g cm−3 | ||
| Melting point | 2 °C; 35 °F; 275 K | ||
| Boiling point | 114 °C; 237 °F; 387 K | ||
| miscible | |||
| log P | 0.67 | ||
| Vapor pressure | 1 kP (at 30.7 °C) | ||
| Acidity (pKa) | 8.10 (N2H5+) | ||
| Basicity (pKb) | 5.90 | ||
|
Refractive index (nD)
|
1.46044 (at 22 °C) | ||
| Viscosity | 0.876 cP | ||
| Structure | |||
| Triangular pyramidal at N | |||
| 1.85 D | |||
| Thermochemistry | |||
|
Std molar
entropy (S |
121.52 J K−1 mol−1 | ||
|
Std enthalpy of
formation (ΔfH |
50.63 kJ mol−1 | ||
| Hazards | |||
| Safety data sheet | ICSC 0281 | ||
| GHS pictograms |
    
|
||
| GHS signal word | DANGER | ||
| H226, H301, H311, H314, H317, H331, H350, H410 | |||
| P201, P261, P273, P280, P301+310, P305+351+338 | |||
|
EU classification (DSD)
|
|
||
| R-phrases | R45, R10, R23/24/25, R34, R43, R50/53 | ||
| S-phrases | S53, S45, S60, S61 | ||
| NFPA 704 | |||
| Flash point | 52 °C (126 °F; 325 K) | ||
| 24 to 270 °C (75 to 518 °F; 297 to 543 K) | |||
| Explosive limits | 1.8–99.99% | ||
| Lethal dose or concentration (LD, LC): | |||
|
LD50 (median dose)
|
59–60 mg/kg (oral in rats, mice) | ||
|
LC50 (median concentration)
|
260 ppm (rat, 4 hr) 630 ppm (rat, 1 hr) 570 ppm (rat, 4 hr) 252 ppm (mouse, 4 hr) |
||
| US health exposure limits (NIOSH): | |||
|
PEL (Permissible)
|
TWA 1 ppm (1.3 mg/m3) [skin] | ||
|
REL (Recommended)
|
Ca C 0.03 ppm (0.04 mg/m3) [2-hour] | ||
|
IDLH (Immediate danger)
|
Ca [50 ppm] | ||
| Related compounds | |||
|
Other anions
|
tetrafluorohydrazine hydrogen peroxide diphosphane diphosphorus tetraiodide |
||
|
Other cations
|
organic hydrazines | ||
|
Related Binary azanes
|
Ammonia triazane |
||
|
Related compounds
|
diazene triazene tetrazene diphosphene |
||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
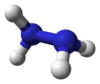
Hydrazine is an inorganic compound with the chemical formula N
2H
4 (also written H
2NNH
2). A simple pnictogen hydride, it is a colorless flammable liquid with an ammonia-like odor. Hydrazine is highly toxic and dangerously unstable unless handled in solution. As of 2000[update], approximately 120,000 tons of hydrazine hydrate (corresponding to a 64% solution of hydrazine in water by weight) were manufactured worldwide per year. Hydrazine is mainly used as a foaming agent in preparing polymer foams, but significant applications also include its uses as a precursor to polymerization catalysts and pharmaceuticals. Additionally, hydrazine is used in various rocket fuels and to prepare the gas precursors used in air bags. Hydrazine is used within both nuclear and conventional electrical power plant steam cycles as an oxygen scavenger to control concentrations of dissolved oxygen in an effort to reduce corrosion. Anhydrous hydrazine is corrosive towards glass, in a manner similar to hydrofluoric acid.
Each H2N−N subunit is pyramidal in shape. The N−N single bond distance is 1.45 Å (145 pm), and the molecule adopts a gauche conformation. The rotational barrier is twice that of ethane. These structural properties resemble those of gaseous hydrogen peroxide, which adopts a "skewed" anticlinal conformation, and also experiences a strong rotational barrier.
...
Wikipedia