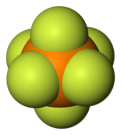
Hexafluorophosphate
|
|
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
Hexafluorophosphate
|
|||
|
Systematic IUPAC name
Hexafluoro-λ5-phosphanuide (substitutive)
Hexafluoridophosphate(1-) (additive) |
|||
| Identifiers | |||
| 3D model (Jmol) | Interactive image | ||
| ChEBI |
CHEBI:30201 |
||
| ChEMBL |
ChEMBL181124 |
||
| ChemSpider |
9502 |
||
| 2704 | |||
| PubChem | 9886 | ||
|
|||
|
|||
| Properties | |||
| [PF6]− | |||
| Molar mass | 144.964181 g mol−1 | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Hexafluorophosphate is an anion with chemical formula of PF−
6. This octahedral species is isoelectronic with sulfur hexafluoride, SF6, and the hexafluorosilicate dianion, SiF62−, and is valence isoelectronic with the highly stable superacid anion fluoroantimonate SbF−
6. As a non-coordinating anion, it is a poor nucleophile. It is prone to decomposition with the release of hydrogen fluoride in ionic liquids but is generally extremely stable in solution. Hydrolysis to the phosphate ion is very slow even in concentrated acid with warming, and even slower under basic conditions.
Hexafluorophosphate salts can be prepared by the reaction of phosphorus pentachloride and alkali or ammonium halide in a solution of hydrofluoric acid:
Hexafluorophosphoric acid can be prepared by direct reaction of hydrogen fluoride with phosphorus pentafluoride. It is a strong Brønsted acid that is typically generated in situ immediately prior to its use.
...
Wikipedia