Phosphate
 |
|||
|
|
|||
| Names | |||
|---|---|---|---|
|
Systematic IUPAC name
Phosphate
|
|||
| Identifiers | |||
|
14265-44-2 |
|||
| 3D model (Jmol) |
Interactive image Interactive image Interactive image |
||
| 3903772 | |||
| ChEBI |
CHEBI:18367 |
||
| ChemSpider |
1032 |
||
| ECHA InfoCard | 100.110.746 | ||
| 1997 | |||
| MeSH | Phosphates | ||
| PubChem | [https://pubchem.ncbi.nlm.nih.gov/compound/1061 | ||
| UNII |
NK08V8K8HR |
||
|
|||
|
|||
| Properties | |||
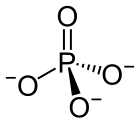
| PO3− 4 |
|||
| Molar mass | 94.9714 g mol−1 | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
pubchem from PubChem 1061 pubchem from PubChem]
A phosphate (PO3−
4) is an inorganic chemical and a salt of phosphoric acid. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Of the various phosphoric acids and phosphates, organic phosphates are important in biochemistry and biogeochemistry (ecology), and inorganic phosphates are mined to obtain phosphorus for use in agriculture and industry. At elevated temperatures in the solid state, phosphates can condense to form pyrophosphates.
In biology, adding phosphates to—and removing them from—proteins in cells are both pivotal in the regulation of metabolic processes. Referred to as phosphorylation and dephosphorylation, respectively, they are important ways that energy is stored and released in living systems.
The phosphate ion is a polyatomic ion with the empirical formula PO3−
4 and a molar mass of 94.97 g/mol. It consists of one central phosphorus atom surrounded by four oxygen atoms in a tetrahedral arrangement. The phosphate ion carries a −3 formal charge and is the conjugate base of the hydrogen phosphate ion, HPO2−
4, which is the conjugate base of H
2PO−
4, the dihydrogen phosphate ion, which in turn is the conjugate base of H
3PO
4, phosphoric acid. A phosphate salt forms when a positively charged ion attaches to the negatively charged oxygen atoms of the ion, forming an ionic compound. Many phosphates are not soluble in water at standard temperature and pressure. The sodium, potassium, rubidium, caesium, and ammonium phosphates are all water-soluble. Most other phosphates are only slightly soluble or are insoluble in water. As a rule, the hydrogen and dihydrogen phosphates are slightly more soluble than the corresponding phosphates. The pyrophosphates are mostly water-soluble.
...
Wikipedia


