Green vitriol
 iron(II) sulfate, when dissolved in water
|
|
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Iron(II) sulfate
|
|
| Other names
Ferrous sulfate, Green vitriol, Iron vitriol, Copperas, Melanterite, Szomolnokite
|
|
| Identifiers | |
|
|
|
3D model (Jmol)
|
|
| ChEBI |
|
| ChemSpider | |
| ECHA InfoCard | 100.028.867 |
| EC Number | 231-753-5 |
|
PubChem CID
|
|
| RTECS number | NO8500000 (anhydrous) NO8510000 (heptahydrate) |
| UNII |
|
| UN number | 3077 |
|
|
|
|
| Properties | |
| FeSO4 | |
| Molar mass | 151.91 g/mol (anhydrous) 169.93 g/mol (monohydrate) 241.99 g/mol (pentahydrate) 260.00 g/mol (hexahydrate) 278.02 g/mol (heptahydrate) |
| Appearance | White crystals (anhydrous) White-yellow crystals (monohydrate) Blue-green crystals (heptahydrate) |
| Odor | Odorless |
| Density | 3.65 g/cm3 (anhydrous) 3 g/cm3 (monohydrate) 2.15 g/cm3 (pentahydrate) 1.934 g/cm3 (hexahydrate) 1.895 g/cm3 (heptahydrate) |
| Melting point | 680 °C (1,256 °F; 953 K) (anhydrous) decomposes 300 °C (572 °F; 573 K) (monohydrate) decomposes 60–64 °C (140–147 °F; 333–337 K) (heptahydrate) decomposes |
| Monohydrate: 44.69 g/100 mL (77 °C) 35.97 g/100 mL (90.1 °C) Heptahydrate: 15.65 g/100 mL (0 °C) 20.5 g/100 mL (10 °C) 29.51 g/100 mL (25 °C) 39.89 g/100 mL (40.1 °C) 51.35 g/100 mL (54 °C) |
|
| Solubility | Negligible in alcohol |
| Solubility in ethylene glycol | 6.4 g/100 g (20 °C) |
| Vapor pressure | 1.95 kPa (heptahydrate) |
|
1.24×10−2 cm3/mol (anhydrous) 1.05×10−2 cm3/mol (monohydrate) 1.12×10−2 cm3/mol (heptahydrate) +10200×10−6 cm3/mol |
|
|
Refractive index (nD)
|
1.591 (monohydrate) 1.526–1.528 (21 °C, tetrahydrate) 1.513–1.515 (pentahydrate) 1.468 (hexahydrate) 1.471 (heptahydrate) |
| Structure | |
|
Orthorhombic, oP24 (anhydrous) Monoclinic, mS36 (monohydrate) Monoclinic, mP72 (tetrahydrate) Triclinic, aP42 (pentahydrate) Monoclinic, mS192 (hexahydrate) Monoclinic, mP108 (heptahydrate) |
|
| Pnma, No. 62 (anhydrous) C2/c, No. 15 (monohydrate, hexahydrate) P21/n, No. 14 (tetrahydrate) P1, No. 2 (pentahydrate) P21/c, No. 14 (heptahydrate) |
|
| 2/m 2/m 2/m (anhydrous) 2/m (monohydrate, tetrahydrate, hexahydrate, heptahydrate) 1 (pentahydrate) |
|
|
a = 8.704(2) Å, b = 6.801(3) Å, c = 4.786(8) Å (293 K, anhydrous)
α = 90°, β = 90°, γ = 90°
|
|
| Octahedral (Fe2+) | |
| Thermochemistry | |
| 100.6 J/mol·K (anhydrous) 394.5 J/mol·K (heptahydrate) |
|
|
Std molar
entropy (S |
107.5 J/mol·K (anhydrous) 409.1 J/mol·K (heptahydrate) |
|
Std enthalpy of
formation (ΔfH |
−928.4 kJ/mol (anhydrous) −3016 kJ/mol (heptahydrate) |
|
Gibbs free energy (ΔfG˚)
|
−820.8 kJ/mol (anhydrous) −2512 kJ/mol (heptahydrate) |
| Pharmacology | |
| B03AA07 (WHO) | |
| Hazards | |
| GHS pictograms |  |
| GHS signal word | Warning |
| H302, H315, H319 | |
| P305+351+338 | |
|
EU classification (DSD)
|
|
| R-phrases | R22, R36/38 |
| S-phrases | (S2), S46 |
| NFPA 704 | |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose)
|
237 mg/kg (rat, oral) |
| US health exposure limits (NIOSH): | |
|
REL (Recommended)
|
TWA 1 mg/m3 |
| Related compounds | |
|
Other cations
|
Cobalt(II) sulfate Copper(II) sulfate Manganese(II) sulfate Nickel(II) sulfate |
|
Related compounds
|
Iron(III) sulfate |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
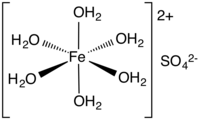
Iron(II) sulfate (British English: iron(II) sulphate) or ferrous sulfate denotes a range of salts with the formula FeSO4·xH2O. These compounds exist most commonly as the heptahydrate (x = 7) but are known for several values of x. The hydrated form is used medically to treat iron deficiency, and also for industrial applications. Known since ancient times as copperas and as green vitriol, the blue-green heptahydrate is the most common form of this material. All the iron(II) sulfates dissolve in water to give the same aquo complex [Fe(H2O)6]2+, which has octahedral molecular geometry and is paramagnetic. The name copperas dates from times when the copper(II) sulfate was known as blue copperas, and perhaps in analogy, iron(II) and zinc sulfate were known respectively as green and white copperas.
It is on the World Health Organization's List of Essential Medicines, the most important medications needed in a basic health system.
Industrially, ferrous sulfate is mainly used as a precursor to other iron compounds. It is a reducing agent, and as such is useful for the reduction of chromate in cement to less toxic Cr(III) compounds. Historically ferrous sulfate was used in the textile industry for centuries as a dye fixative. It is used historically to blacken leather and as a constituent of ink.
Together with other iron compounds, ferrous sulfate is used to fortify foods and to treat and prevent iron deficiency anemia. Constipation is a frequent and uncomfortable side effect associated with the administration of oral iron supplements. Stool softeners often are prescribed to prevent constipation.
Ferrous sulfate was used in the manufacture of inks, most notably iron gall ink, which was used from the middle ages until the end of the eighteenth century. Chemical tests made on the Lachish letters (c.588–586 BCE) showed the possible presence of iron. It is thought that oak galls and copperas may have been used in making the ink on those letters. It also finds use in wool dyeing as a mordant. Harewood, a material used in marquetry and parquetry since the 17th century, is also made using ferrous sulfate.
...
Wikipedia

