Gold(III) chloride
 |
|
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Gold(III) trichloride
|
|
| Other names
Auric chloride
Gold trichloride |
|
| Identifiers | |
|
13453-07-1 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:30076 |
| ChemSpider |
24244 |
| ECHA InfoCard | 100.033.280 |
| PubChem | 26030 |
| RTECS number | MD5420000 |
| UNII |
15443PR153 |
|
|
|
|
| Properties | |
| AuCl3 (exists as Au2Cl6) |
|
| Molar mass | 303.325 g/mol |
| Appearance | Red crystals (anhydrous); golden, yellow crystals (monohydrate) |
| Density | 4.7 g/cm3 |
| Melting point | 254 °C (489 °F; 527 K) (decomposes) |
| 68 g/100 ml (cold) | |
| Solubility | soluble in ether, slightly soluble in liquid ammonia |
| −112·10−6 cm3/mol | |
| Structure | |
| monoclinic | |
| Square planar | |
| Hazards | |
| Main hazards | Irritant |
| Safety data sheet | See: data page |
| R-phrases | R36/37/38 |
| S-phrases | S26 S36 |
| Related compounds | |
|
Other anions
|
Gold(III) fluoride Gold(III) bromide |
|
Other cations
|
Gold(I) chloride Silver(I) chloride Platinum(II) chloride Mercury(II) chloride |
| Supplementary data page | |
|
Refractive index (n), Dielectric constant (εr), etc. |
|
|
Thermodynamic
data |
Phase behaviour solid–liquid–gas |
| UV, IR, NMR, MS | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Gold(III) chloride, traditionally called auric chloride, is a chemical compound of gold and chlorine. With the molecular formula Au2Cl6, the name gold trichloride is a simplification, referring to the empirical formula, AuCl3. The Roman numerals in the name indicate that the gold has an oxidation state of +3, which is common for gold compounds. There is also another related chloride of gold, gold(I) chloride (AuCl). Chloroauric acid, HAuCl4, the product formed when gold dissolves in aqua regia, is sometimes referred to as "gold chloride" or "acid gold trichloride". Gold(III) chloride is very hygroscopic and highly soluble in water as well as ethanol. It decomposes above 160 °C or in light.
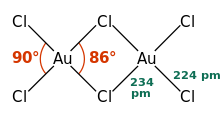
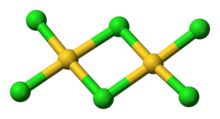
AuCl3 exists as a chloride-bridged both as a solid and as a vapour, at least at low temperatures.Gold(III) bromide behaves analogously. The structure is similar to that of iodine(III) chloride.
In gold(III) chloride, each gold center is square planar, which is typical of a metal complex with a d8 electron count. The bonding in AuCl3 is considered somewhat covalent.
Gold(III) chloride is most often prepared by passing chlorine gas over gold powder at 180 °C:
Another method of preparation is by reacting Au3+ species with chloride to produce tetrachloroaurate. Its acid, chloroauric acid, is then heated to eliminate hydrogen chloride gas. Reaction with aqua regia produces gold(III) chloride:
...
Wikipedia
