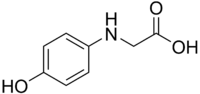
Glycin
 |
|
| Names | |
|---|---|
|
IUPAC name
2-(4-hydroxyphenyl)aminoacetic acid
|
|
| Other names
N-(4-hydroxyphenyl)glycine
p-hydroxyanilinoacetic acid photoglycine |
|
| Identifiers | |
|
122-87-2 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:55443 |
| ChemSpider |
60494 |
| ECHA InfoCard | 100.004.165 |
| PubChem | 67149 |
|
|
|
|
| Properties | |
| C8H9NO3 | |
| Molar mass | 167.16 g/mol |
| Appearance | brown powder |
| Density | 1.411 g/mL |
| Melting point | 244 °C (471 °F; 517 K) |
| Boiling point | 446.3 °C (835.3 °F; 719.5 K) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Glycin, or N-(4-hydroxyphenyl)glycine, is N-substituted p-aminophenol. It is a photographic developing agent used in classic black-and-white developer solutions. It is unrelated to the amino acid glycine. When fresh, it is typically characterized as thin plates of white or silvery powder, turning brown with age. It is sparingly soluble in water and most organic solvents; it is readily soluble in alkalies and acids.
Glycin is related to 4-aminophenol and Metol. Compared to Metol, glycin has a carboxyl group attached to the methyl group of the Metol. This weakens the reduction potential of the compound, and the two developers have markedly different character. Glycin is slower-acting, but much longer-lasting in solution. Glycin is rarely used as a developing agent today, primarily because it is expensive and manufactured for specialty applications only. In its dry form, it also has limited shelf life compared to Metol and Phenidone.
Glycin can be synthesized by reacting p-aminophenol with chloracetic acid in a solvent.
Other uses of glycin can be found in some procedures of analytical chemistry.
...
Wikipedia
