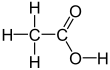
Glacial acetic acid
|
|
|||
|
|
|||
 |
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
Acetic acid
|
|||
|
Systematic IUPAC name
Ethanoic acid
|
|||
| Other names
Vinegar (when dilute); Hydrogen acetate; Methanecarboxylic acid
|
|||
| Identifiers | |||
|
3D model (JSmol)
|
|||
| 3DMet | B00009 | ||
| Abbreviations | AcOH | ||
| 506007 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.528 | ||
| EC Number | 200-580-7 | ||
| E number | E260 (preservatives) | ||
| 1380 | |||
| KEGG | |||
| MeSH | Acetic+acid | ||
|
PubChem CID
|
|||
| RTECS number | AF1225000 | ||
| UNII | |||
| UN number | 2789 | ||
|
|||
|
|||
| Properties | |||
| C2H4O2 | |||
| Molar mass | 60.05 g·mol−1 | ||
| Appearance | Colourless liquid | ||
| Odor | Pungent/Vinegar-like | ||
| Density | 1.049 g cm−3 (l); 1.27 g cm cm−3 (s) | ||
| Melting point | 16 to 17 °C; 61 to 62 °F; 289 to 290 K | ||
| Boiling point | 118 to 119 °C; 244 to 246 °F; 391 to 392 K | ||
| Miscible | |||
| log P | -0.322 | ||
| Acidity (pKa) | 4.76 | ||
| Basicity (pKb) | 9.24 (basicity of acetate ion) | ||
| -31.54·10−6 cm3/mol | |||
|
Refractive index (nD)
|
1.371 | ||
| Viscosity | 1.22 mPa s | ||
| 1.74 D | |||
| Thermochemistry | |||
| 123.1 J K−1 mol−1 | |||
|
Std molar
entropy (S |
158.0 J K−1 mol−1 | ||
|
Std enthalpy of
formation (ΔfH |
-483.88—483.16 kJ mol−1 | ||
|
Std enthalpy of
combustion (ΔcH |
-875.50—874.82 kJ mol−1 | ||
| Pharmacology | |||
| G01AD02 (WHO) S02AA10 (WHO) | |||
| Hazards | |||
| Safety data sheet | See: data page | ||
| GHS pictograms |
 
|
||
| GHS signal word | Danger | ||
| H226, H314 | |||
| P280, P305+351+338, P310 | |||
| NFPA 704 | |||
| Flash point | 40 °C (104 °F; 313 K) | ||
| 427 °C (801 °F; 700 K) | |||
| Explosive limits | 4–16% | ||
| Lethal dose or concentration (LD, LC): | |||
|
LD50 (median dose)
|
3.31 g kg−1, oral (rat) | ||
|
LC50 (median concentration)
|
5620 ppm (mouse, 1 hr) 16000 ppm (rat, 4 hr) |
||
| US health exposure limits (NIOSH): | |||
|
PEL (Permissible)
|
TWA 10 ppm (25 mg/m3) | ||
|
REL (Recommended)
|
TWA 10 ppm (25 mg/m3) ST 15 ppm (37 mg/m3) | ||
|
IDLH (Immediate danger)
|
50 ppm | ||
| Related compounds | |||
|
Related carboxylic acids
|
Formic acid Propionic acid |
||
|
Related compounds
|
Acetaldehyde Acetamide |
||
| Supplementary data page | |||
|
Refractive index (n), Dielectric constant (εr), etc. |
|||
|
Thermodynamic
data |
Phase behaviour solid–liquid–gas |
||
| UV, IR, NMR, MS | |||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Acetamide
Acetic anhydride
Acetonitrile
Acetyl chloride
Ethanol
Ethyl acetate
Potassium acetate
Sodium acetate
Thioacetic acid
Acetic acid /əˈsiːtɪk/, systematically named ethanoic acid /ˌɛθəˈnoʊɪk/, is a colourless liquid organic compound with the chemical formula CH3COOH (also written as CH3CO2H or C2H4O2). When undiluted, it is sometimes called glacial acetic acid. Vinegar is roughly 3–9% acetic acid by volume, making acetic acid the main component of vinegar apart from water. Acetic acid has a distinctive sour taste and pungent smell. In addition to household vinegar, it is mainly produced as a precursor to polyvinyl acetate and cellulose acetate. It is classified as a weak acid since it only partially dissociates in solution, but concentrated acetic acid is corrosive and can attack the skin.
...
Wikipedia