Genistein
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
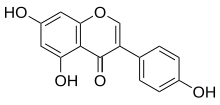
5,7-Dihydroxy-3-(4-hydroxyphenyl)chromen-4-one
|
|
| Other names
4',5,7-Trihydroxyisoflavone
|
|
| Identifiers | |
|
446-72-0 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:28088 |
| ChEMBL |
ChEMBL44 |
| ChemSpider |
4444448 |
| DrugBank |
DB01645 |
| ECHA InfoCard | 100.006.524 |
| 2826 | |
| KEGG |
C06563 |
| PubChem | 5280961 |
| UNII |
DH2M523P0H |
|
|
|
|
| Properties | |
| C15H10O5 | |
| Molar mass | 270.24 g·mol−1 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Genistein is an isoflavone that is described as an angiogenesis inhibitor and a phytoestrogen. It was first isolated in 1899 from the dyer's broom, Genista tinctoria; hence, the chemical name. The compound structure was established in 1926, when it was found to be identical with that of prunetol. It was chemically synthesized in 1928.
Isoflavones such as genistein and daidzein are found in a number of plants including lupin, fava beans, soybeans, kudzu, and psoralea being the primary food source, also in the medicinal plants, Flemingia vestita and F. macrophylla, and coffee. It can also be found in Maackia amurensis cell cultures.
Most of the isoflavones in plants are present in a glycosylated form. The unglycosylated aglycones can be obtained through various means such as treatment with the enzyme β-glucosidase, acid treatment of soybeans followed by solvent extraction, or by chemical synthesis. Acid treatment is a harsh method as concentrated inorganic acids are used. Both enzyme treatment and chemical synthesis are costly. A more economical process consisting of fermentation for in situ production of β-glucosidase to isolate genistein has been recently investigated.
Besides functioning as antioxidant and anthelmintic, many isoflavones have been shown to interact with animal and human estrogen receptors, causing effects in the body similar to those caused by the hormone estrogen. Isoflavones also produce non-hormonal effects.
...
Wikipedia
