Gallium(III) oxide
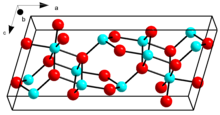 Crystal structure of β-Ga2O3
|
|
| Names | |
|---|---|
| Other names
gallium trioxide, gallium sesquioxide
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.031.525 |
|
PubChem CID
|
|
| RTECS number | LW9650000 |
|
|
|
|
| Properties | |
| Ga2O3 | |
| Molar mass | 187.444 g/mol |
| Appearance | white crystalline powder |
| Density | 6.44 g/cm3, alpha 5.88 g/cm3, beta |
| Melting point | 1,900 °C (3,450 °F; 2,170 K) alpha 1725 °C, beta |
| insoluble | |
| Solubility | soluble in most acids |
| Structure | |
| α: Trigonal, hR30, space group = R3c, No. 167 β: Monoclinic, mS20, space group = C2/m, No. 12 |
|
|
a = 0.49835 / 1.22247 nm, b = 0.49835 / 0.30403 nm, c = 0.53286 / 0.58088 nm
|
|
|
Formula units (Z)
|
6 / 4 |
| Thermochemistry | |
| 92.1 J/mol·K | |
|
Std molar
entropy (S |
85.0 J/mol·K |
|
Std enthalpy of
formation (ΔfH |
−1089.1 kJ/mol |
|
Gibbs free energy (ΔfG˚)
|
-998.3 kJ/mol |
| Hazards | |
|
EU classification (DSD) (outdated)
|
not listed |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
β: Monoclinic, mS20, space group = C2/m, No. 12
Gallium(III) oxide is an inorganic compound with the formula Ga2O3. It exists as several polymorphs, all of which are white, water-insoluble solids. Although no commercial applications exist, Ga2O3 is an intermediate in the purification of gallium, which is consumed almost exclusively as gallium arsenide.
Gallium oxide is precipitated in hydrated form upon neutralization of acidic or basic solution of gallium salt. Also, it is formed on heating gallium in air or by thermally decomposing gallium nitrate at 200–250 ˚C. It can occur in five different modifications, α, β, γ, δ, and ε. Of these modifications β-Ga2O3 is the most stable form.
Gallium(III) oxide is amphoteric. It reacts with alkali metal oxides at high temperature to form e.g. NaGaO2, and with Mg, Zn, Co, Ni, Cu oxides to form spinels, e.g. MgGa2O4. It dissolves in strong alkali to form a solution of the gallate ion, Ga(OH)−
4.
With HCl, it forms gallium trichloride GaCl3.
It can be reduced to gallium suboxide (gallium(I) oxide) Ga2O by H2. or by reaction with gallium metal
β-Ga2O3, with a melting point of 1900 ˚C, is the most stable crystalline modification. The oxide ions are in a distorted cubic closest packing arrangement, and the gallium (III) ions occupy distorted tetrahedral and octahedral sites, with Ga-O bond distances of 1.83 and 2.00 Å respectively.
...
Wikipedia
