Formins
| DRF Autoregulatory Domain |

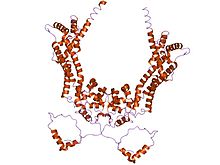
crystal structure of the n-terminal mdia1 armadillo repeat region and dimerisation domain in complex with the mdia1 autoregulatory domain (dad)
|
| Identifiers |
| Symbol |
Drf_DAD |
| Pfam |
PF06345 |
| InterPro |
IPR010465 |
|
|
Formins (formin homology proteins) are a group of proteins that are involved in the polymerization of actin and associate with the fast-growing end (barbed end) of actin filaments. Most formins are Rho-GTPase effector proteins. Formins regulate the actin and microtubule cytoskeleton and are involved in various cellular functions such as cell polarity, cytokinesis, cell migration and SRF transcriptional activity. Formins are multidomain proteins that interact with diverse signalling molecules and cytoskeletal proteins, although some formins have been assigned functions within the nucleus.
Formins have been found in all eukaryotes studied. In humans, 15 different formin proteins are present that have been classified in 7 subgroups. By contrast, yeasts contain only 2-3 formins.
Formins are characterised by the presence of three formin homology (FH) domains (FH1, FH2 and FH3), although members of the formin family do not necessarily contain all three domains. In addition, other domains are usually present, such as PDZ, DAD, WH2, or FHA domains.
The proline-rich FH1 domain mediates interactions with a variety of proteins, including the actin-binding protein profilin, SH3 (Src homology 3) domain proteins, and WW domain proteins. The actin nucleation-promoting activity of S. cerevisiae formins has been localized to the FH2 domain. The FH2 domain is required for the self-association of formin proteins through the ability of FH2 domains to directly bind each other, and may also act to inhibit actin polymerisation. The FH3 domain is less well conserved and is required for directing formins to the correct intracellular location, such the mitotic spindle, or the projection tip during conjugation. In addition, some formins can contain a GTPase-binding domain (GBD) required for binding to Rho small GTPases, and a C-terminal conserved DRF autoregulatory domain (Dia-autoregulatory domain) (DAD). The GBD domain is a bifunctional autoinhibitory domain that interacts with and is regulated by activated Rho family members. Mammalian Drf3 contains a CRIB-like motif within its GBD for binding to Cdc42, which is required for Cdc42 to activate and guide Drf3 towards the cell cortex where it remodels the actin skeleton. The DRF autoregulatory domain binds the N-terminal GTPase-binding domain; this link is broken when GTP-bound Rho binds to the GBD and activates the protein. The addition of the DAD to mammalian cells induces actin filament formation, stabilises microtubules, and activates serum-response mediated transcription. Another commonly found domain is an armadillo repeat region (ARR) located in the FH3 domain.
...
Wikipedia